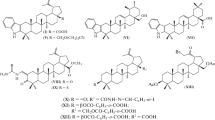
Novel triterpene homodimers linked through ring A C-2 by a 1,3-diyne spacer were synthesized via Pd/Cu-catalyzed acetylene homocoupling of C-2 propynyl derivatives of betulinic and ursolic acid aminocarboxamides. The cytotoxic activities of the synthesized compounds against a broad panel of tumor cell lines were assessed. High antiproliferative effects were found for monomeric betulinic and ursolic acid aminocarboxamides. Bis-derivatives of the triterpene acids exhibited much lower antitumor activities than the corresponding starting monomers.
Similar content being viewed by others
References
R. A. Hill and J. D. Connolly, Nat. Prod. Rep., 29, 1028 (2015).
J. Sarek, M. Kvasnica, M. Vlk, M. Urban, P. Dzubak, and M. Hajduch, in: Research on Melanoma – A Glimpse into Current Directions and Future Trends, M. Murph (ed.), IntechOpen, 2011, Chap. 7, p. 125.
R. H. Cichewicz and S. A. Kouzi, Med. Res. Rev., 24, 90 (2004).
J. A. R. Salvador, V. M. Moreira, B. M. F. Goncalves, A. S. Leal, and Y. Jing, Nat. Prod. Rep., 29, 1463 (2012).
H. Chen, Y. Gao, A. Wang, X. Zhou, Y. Zheng, and J. Zhou, Eur. J. Med. Chem., 92, 648 (2015).
A. Y. Spivak, Z. R. Galimshina, D. A. Nedopekina, and V. N. Odinokov, Chem. Nat. Compd., 54, 265 (2018).
A. Y. Spivak, R. Khalitova, D. Nedopekina, L. Dzhemileva, M. Yunusbaeva, V. Odinokov, V. D’yakonov, and U. Dzhemilev, Molecules, 23, 3000 (2018).
M. Kahnt, L. F. N. Heller, A. Al-Harrasi, and R. Csuk, Molecules, 23, 1 (2018).
M. Kahnt, A. Loesche, I. Serbian, S. Hoenke, L. Fischer, A. Al-Harrasi, and R. Csuk, Steroids, 149, 108422 (2019).
M. Hadden and B. Blagg, Anticancer Agents Med. Chem., 8, 807 (2008).
B. Bednarczyk-Cwynar and A. Gunther, Curr. Med. Chem., 24, 2205 (2017).
A. Y. Spivak, R. R. Gubaidullin, Z. R. Galimshina, D. A. Nedopekina, and V. N. Odinokov, Tetrahedron, 72, 1249 (2016).
H. A. Stefani, A. S. Guarezemini, and R. Cella, Tetrahedron, 66, 7871 (2010).
A. Y. Spivak, D. A. Nedopekina, Z. R. Galimshina, R. R. Khalitova, Z. R. Sadretdinova, R. R. Gubaidullin, and V. N. Odinokov, ARKIVOC, 7, 1 (2018).
Acknowledgment
The work was financially supported by the Russian Science Foundation (Grant No. 19-73-00155). We thank the National Cancer Institute for in vitro tests for antitumor activity of the synthesized compounds. Structural studies of the synthesized compounds were performed using the Agidel Common Use Center, Ufa FRC, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2021, pp. 103–111.
Rights and permissions
About this article
Cite this article
Spivak, A.Y., Khalitova, R.R., Gubaidullin, R.R. et al. Synthesis and Cytotoxic Activity of Monomeric and Dimeric Aminocarboxamides of Betulinic and Ursolic Acids. Chem Nat Compd 57, 123–132 (2021). https://doi.org/10.1007/s10600-021-03296-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03296-z