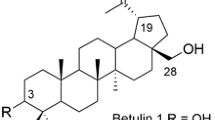
O- and N-propylamino-derivatives of betulin and betulonic acid oxime and methyl ester were synthesized. Their antitumor activity against a panel of 60 human cancer cell lines was studied. Two betulin derivatives, 3β-hydroxy- 4 and 3β-O-(3-propylamino)-28-O-(3,6-diaminohexyl)lupa-20(29)-ene 5, exhibited antitumor activity against most of 60 cancer cell lines of nine different human tumor types. They were more active than doxorubicin against HCT-15 colon cancer and NCI/ADR-RES ovary cancer cells.
Similar content being viewed by others
References
A. L. Harvey, Drug. Discovery Today, 13, 894 (2008).
J. A. R. Salvador, A. S. Leal, A. S. Valdeira, B. M. F. Goncalves, D. P. S. Alho, S. A. C. Figueiredo, S. M. Silvestre, and V. I. S. Mendes, Eur. J. Med. Chem., 142, 95 (2017).
W. J. Shan, L. W. Zhang, J. G. Xiang, and Z. J. Zhan, Chem. Biodiv., 10, 1392 (2013).
J. Sarek, M. Kvasnica, M. Vlk, M. Urban, P. Dzubak, and M. Hajduchy, The Potential of Triterpenoids in the Treatment of Melanoma, IntechOpen, Vukovar, 2011, 35 pp.
L. Ma, X. Wang, W. Li, D. Miao, Y. Li, J. Lu, and Y. Zhao, Eur. J. Med. Chem., 187, 111964 (2020).
S. H. Safe, P. L. Prather, L. K. Brents, G. Chadalapaka, and I. Jutooru, Anti-Cancer Agents Med. Chem., 12, 1211 (2012).
J. S. Pyo, S. H. Roh, D. K. Kim, J. G. Lee, and Y. Y. Lee, Planta Med., 75, 127 (2009).
R. Csuk, A. Barthel, R. Kluge, and D. Strohl, Bioorg. Med. Chem., 18, 7252 (2010).
H. Kommera, G. N. Kaluderovic, S. Dittrich, J. Kalbitz, B. Drager, T. Muller, and R. Paschke, Bioorg. Med. Chem. Lett., 20, 3409 (2010).
O. B. Kazakova, G. V. Giniyatullina, A. G. Mustafin, D. A. Babkov, E. V. Sokolova, and A. A. Spasov, Molecules, 25, 4833 (2020).
G. V. Giniyatullina, O. B. Flekhter, and G. A. Tolstikov, Mendeleev Commun., 19, 32 (2009).
G. V. Giniyatullina and O. B. Kazakova, Chem. Nat. Compd., 54, 913 (2018).
G. V. Giniyatullina, I. E. Smirnova, O. B. Kazakova, N. P. Yavorskaya, I. S. Golubeva, O. S. Zhukova, R. B. Pugacheva, G. N. Apryshko, and V. V. Poroikov, Med. Chem. Res., 24, 3423 (2015).
A. N. Antimonova, N. V. Uzenkova, N. I. Petrenko, M. M. Shakirov, E. E. Shults, and G. A. Tolstikov, Russ. J. Org. Chem., 47, 589 (2011).
O. B. Kazakova, G. V. Giniyatullina, and G. A. Tolstikov, Russ. J. Bioorg. Chem., 37, 619 (2011).
G. V. Giniyatullina, O. B. Kazakova, N. I. Medvedeva, I. V. Sorokina, N. A. Zhukova, T. G. Tolstikova, and G. A. Tolstikov, Russ. J. Bioorg. Chem., 39, 329 (2013).
G. V. Giniyatullina, O. B. Kazakova, N. I. Medvedeva, G. A. Tolstikov, and G. N. Apryshko, Russ. J. Bioorg. Chem., 40, 198 (2014).
O. B. Kazakova, G. V. Giniyatullina, G. A. Tolstikov, I. P. Baikova, L. Zaprutko, and G. N. Apryshko, Russ. J. Bioorg. Chem., 37, 369 (2011).
O. V. Savinova, N. I. Pavlova, and E. I. Boreko, Antibiot. Khimioter., 54, 5 (2009).
E. I. Boreko, N. I. Pavlova, O. V. Savinova, O. B. Flekhter, L. R. Nigmatullina, L. A. Baltina, F. Z. Galin, and G. A. Tolstikov, Rep. Belarus Pat. No. 7, 811 (2005).
G. V. Giniyatullina, A. G. Mustafin, and O. B. Kazakova, Chem. Nat. Compd., 56, 92 (2020).
L. F. Fieser and M. Fieser, Reagents for Organic Synthesis, Wiley, New York, 1967, 1457 pp.
E. Dunkelblum, Tetrahedron, 28, 3879 (1972).
L. Pohjala, S. Alakurtti, T. Ahola, J. Yli-Kauhaluoma, and P. Tammela, J. Nat. Prod., 72, 1917 (2009).
M. R. Boyd and K. D. Paul, Drug Res. Rep., 34, 91 (1995).
M. R. Grever, S. A. Schepartz, and B. A. Chabner, Semin. Oncol., 19, 622 (1992).
A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. D. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-Wolff, M. Gray-Goodrich, H. Campbell, J. Mayo, and M. J. Boyd, J. Nat. Cancer Inst., 83, 757 (1991).
A. Monks, D. A. Scudiero, G. S. Johnson, K. D. Paull, and E. A. Sausville, Anti-Cancer Drug Des., 12, 533 (1997).
J. N. Weinstein, T. G. Myers, P. M. O’Connor, S. H. Friend, A. J. Fornace, Jr., K. W. Kohn, T. Fojo, S. E. Bates, L. V. Rubinstein, N. L. Anderson, J. K. Buolamwini, W. W. van Osdol, A. P. Monks, D. A. Scudiero, E. A. Sausville, D. W. Zaharevitz, B. Bunow, V. N. Viswanadhan, G. S. Johnson, R. E. Wittes, and K. D. Paull, Science, 275, 343 (1997).
A. Montoya, J. Quiroga, R. Abonia, M. Nogueras, J. Cobo, and B. Insuasty, Molecules, 19, 18656 (2014).
S. A. F. Rostom, Bioorg. Med. Chem., 14, 6475 (2006).
Acknowledgment
The work was performed on a topic of State Task No. 122031400261-4. We thank the National Cancer Institute for determining the antitumor activity in vitro of 2, 4, 5, 7, 11, 12, 14, and 15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2022, pp. 580–587.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giniyatullina, G.V. Synthesis and Antitumor Activity of O- and N-Propylamino-Derivatives of Betulin. Chem Nat Compd 58, 684–692 (2022). https://doi.org/10.1007/s10600-022-03769-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03769-9