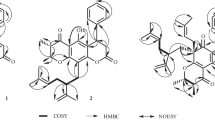
The structure of a new compound was determined using PMR and 13C NMR spectroscopy (HHCOSY, HSBC, HMBC, ROESY) as 2-[3′-methoxy,4-O-β-D-galactopyranos-1-yl)benzyl]-3-(3″,4″-dimethoxybenzyl)-4hydroxybutyric acid, which was isolated for the first time from seeds of Scotch thistle Onopordum acanthium L.


Similar content being viewed by others
References
L. M. Khalilov, A. Z. Khalilova, E. R. Shakurova, I. F. Nuriev, V. V. Kachala, A. S. Shashkov, and U. M. Dzhemelev, Khim. Prir. Soedin., 223 (2003).
A. Z. Khalilova, I. A. Litvinov, D. V. Beskrovnyi, A. T. Gubaidullin, E. R. Shakurova, I. F. Nuriev, L. M. Khalilov, and U. M. Dzhemilev, Khim. Prir. Soedin., 215 (2004).
Plant Resources of the USSR. Flowering Plants, Their Chemical Composition, and Use. Asteraceae Family [in Russian], Nauka, St. Petersburg (1993).
V. N. Kortikov and A. V. Kortikov, Secrets of Healing Herbs [in Russian], Vol. 1, Minsk (1995).
T. A. Goncharova, Encyclopedia of Medicinal Plants (Herbal Treatment) [in Russian], Vol. 2, Izd. Dom MPS, Moscow (1997).
E. Breitmaier and W. Voelter, 13 C NMR Spectroscopy: Methods and Application, Verlag Chemie GmbH, Weinheim/Bergstr (1974).
V. A. Kurkin, Khim. Prir. Soedin., 87 (2003).
B. K. Nakano, K. Nishizawa, I. Takemoto, K. Murakami, and T. Tomimatsu, Phytochemistry, 29, 301 (1989).
G. G. Zapesochnaya, V. A. Kurkin, T. V. Kudryavtseva, B. S. Karasartov, K. S. Cholponbaev, N. A. Tyukavkina, and V. E. Ruchkin, Khim. Prir. Soedin., 40 (1992).
V. A. Kurkin, Phenylpropanoids, Promising Natural Biolocially Active Compounds [in Russian], Izd. Sam. GMU, Samara (1996).
L. Cometa, I. Tomassini, M. Nicoletti, and S. Pieretti, Fitoterapia, 64, 195 (1993).
H. Ravn and L. Brimer, Phytochemistry, 27, 3433 (1988).
V. V. Plemenkov, Introduction to the Chemistry of Natural Compounds [in Russian], Kazan′ (2001), p. 376.
Acknowledgment
We thank A. Z. Khalilova and A. A. Muldashev for help in collecting and identifying the plant material and Doctor of Chemical Sciences Prof. A. S. Shashkov for supplying the 2D NMR results.
Author information
Authors and Affiliations
Corresponding author
Additional information
*For No. XII, see [1].
Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 53–55, January–February, 2009.
Rights and permissions
About this article
Cite this article
Tyumkina, T.V., Nuriev, I.F., Khalilov, L.M. et al. PMR and 13C NMR spectra of biologically active compounds. XIII.* Structure and stereochemistry of a new phenylpropanoid glycoside isolated from Onopordum acanthium seeds. Chem Nat Compd 45, 61–65 (2009). https://doi.org/10.1007/s10600-009-9254-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-009-9254-9