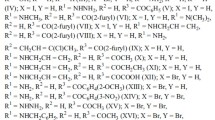Abstract
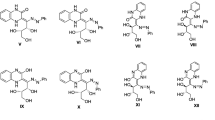
Natural 1,5-di-, 1,4,5-tri-, and 1,4,5,8-tetrahydroxyanthraquinones and their anions and metal complexes were shown to be equilibrium mixtures of tautomers and conformers using quantum-chemical and correlation analysis of elecronic absorption spectra. Solvent effects, ionization, complexation, and the introduction and substitution of substituents were accompanied by shifts of tautomeric and conformational equilibria that determine the color of the compounds.
Similar content being viewed by others
References
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Khim. Prir. Soedin., 119 (2005).
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Khim. Prir. Soedin., 411 (2005).
R. H. Thomson, Naturally Occurring Quinones, II, Academic Press, London-New York (1971); III, Chapman and Hall, London-New York (1987), p. 350.
R. A. Muzychkina, Natural Anthraquinones. Biological Properties and Physicochemical Characteristics, G. A. Tolstikov, ed., Fazis, Moscow (1998).
V. Ya. Fain, 9,10-Anthraquinones and Their Use [in Russian], RAS Photochemistry Center, Moscow (1999).
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Zh. Org. Khim., 41, 43 (2005).
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Zh. Org. Khim., 41, 722 (2005).
M. Dewar, Molecular Orbital Methods in Organic Chemistry [in Russian], Mir, Moscow (1972).
K. Nishimoto and L. S. Forster, Theor. Chim. Acta, 4, 155 (1966).
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Zh. Obshch. Khim., 76, 608 (2006).
S. Gopolakrishnan, S. Neelakantan, and P. V. Raman, J. Indian Chem. Soc., 67, 390 (1990).
V. Ya. Fain, Correlation Analysis of Electronic Absorption Spectra [in Russian], Sputnik+, Moscow (2002).
V. Ya. Fain, Tables of Electronic Absorption Spectra of Anthraquinone and its Derivatives [in Russian], Khimiya, Leningrad (1970).
O. Ekpa, E. Anam, and N. Vethakiyasar, Planta Med., 51, 528 (1985).
V. Ya. Fain, Electronic Absorption Spectra and Structure of 9,10-Anthraquinones. Disubstituted 9,10-Anthraquinones [in Russian], Sputnik+, Moscow (2003).
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Koord. Khim., 30, 712 (2004).
V. Ya. Fain, B. E. Zaitsev, and M. A. Ryabov, Koord. Khim., 32, 143 (2006).
V.Ya. Fain, Dep. ONIITEKhim., Cherkassy (1990), No. 778-khp-90; Ref. Zh. Khim., 9B1251 (1991).
S. Imre, A. Oztinc, and N. Buyuktimkin, Phytochemistry, 13, 681 (1974).
M. Kazmi, A. Malik, S. Hameed, N. Akhtar, and S. N. Ali, Phytochemistry, 36, 761 (1994).
S. Imre, S. Sar, and R. H. Thomson, Phytochemistry, 15, 317 (1976).
E. J. C. Brew and R. H. Thomson, J. Chem. Soc. C, 2007 (1971).
R. Wijnsma and R. Verpoorte, in: Progress in the Chemistry of Organic Natural Products, Springer, Vienna-New York (1986), Vol. 49, p. 79.
H. Brockmann, P. Boldt, and J. Niemeyer, Chem. Ber., 96, 1356 (1963).
H. Brockmann and H. Brockmann, Jr., Chem. Ber., 96, 1771 (1963).
N. P. Mishchenko, L. S. Stepanenko, O. E. Krivoshchekova, and O. B. Maksimov, Khim. Prir. Soedin., 160 (1980).
B. Simoneau and P. Brassard, Tetrahedron, 44, 1015 (1988).
A. Yagi, K. Makino, and I. Nishioka, Chem. Pharm. Bull., 25, 1764 (1977).
K. Chandrasenan, S. Neelakantan, and T. R. Seshadri, Proc. Indian Acad. Sci., Sect. A, 51, 296 (1960).
K. E. Stensio and C. A. Wachtmeister, Acta Chem. Scand., 23, 144 (1969).
N. T. Manojlovic, S. Solujic, S. Sukdolak, and L. J. Krstic, J. Serb. Chem. Soc., 65, 555 (2000).
G. D. Graves and R. Adams, J. Am. Chem. Soc., 45, 2439 (1923).
G. S. R. Rao, T. Hanumaia, and K. V. J. Rao, Indian J. Chem., Sect. B, 19, 97 (1980).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 224–229, May–June, 2006.
Rights and permissions
About this article
Cite this article
Fain, V.Y., Zaitsev, B.E. & Ryabov, M.A. Tautomeric and conformational isomerism of natural hydroxyanthraquinones. Chem Nat Compd 42, 269–276 (2006). https://doi.org/10.1007/s10600-006-0097-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10600-006-0097-3
Key words
- natural anthraquinones
- 1,5-dihydroxyanthraquinone
- 1,4,5-trihydroxyanthraquinone
- 1,4,5,8-tetrahydroxy-2-methylanthraquinone
- anthrarufin
- ziganein
- morindaparvin B
- islandicin
- digitopurpone
- helminthosporin
- erythroglaucin
- xanthorin
- isoxanthorin
- cinnarubin
- ventione A
- cinodontin
- electronic absorption spectra
- quantum-chemical calculation
- substituent σA-constants
- correlation analysis
- prototropic tautomerism
- conformational isomerism
- conformers
- ionization
- metal complexes