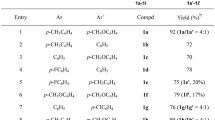
The nitration and halogenation reactions of 2-(pentafluorosulfanyl)- and 2-(trifluoromethyl)-1,3-benzothiazoles were studied. Methods for the preparation of previously undescribed mononitro-substituted 1,3-benzothiazoles (4-nitro-2-(pentafluorosulfanyl)-1,3-benzothiazole, 4-nitro-2-(trifluoromethyl)-1,3-benzothiazole, and 6-nitro-2-(pentafluorosulfanyl)-1,3-benzothiazole) as well as a new method for the synthesis of the previously known 6-nitro-2-(trifluoromethyl)-1,3-benzothiazole were developed. The procedure involved the reaction of 2-(trifluoromethyl)-1,3-benzothiazole with NH4NO3 in TFAA at room temperature. An efficient method for the preparation of 2-substituted 4,5,6,7-tetrabromo-1,3-benzothiazoles based on the reaction of 2-substituted 1,3-benzothiazoles with NBS in TFA–H2SO4 at room temperature was proposed.


Similar content being viewed by others
References
(a) Seth, S. Anti-Inflamm. AntiAllergy Agents Med. Chem. 2015, 14, 98. (b) Gupta, K.; Sirbaiya, A. K.; Kumar, V.; Rahman, M. A. Mini-Rev. Med. Chem. 2022, 22, 1895. (c) Sharma, P. C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D. P. J. Enzyme Inhib. Med. Chem. 2013, 28, 240. (d) Pathak, N.; Rathi, E.; Kumar, N.; Kini, S. G.; Rao, C. M. Mini-Rev. Med. Chem. 2020, 20, 12. (e) Singh, M.; Singh, S. K. Anticancer Agents Med. Chem. 2014, 14, 127.
(a) Welch, J. T. In Fluorine in Pharmaceutical and Medicinal Chemistry. From Biophysical Aspects to Clinical Applications; Gouverneur, V.; Müller, K., Eds.; Imperial College Press: London, 2012, p. 175. (b) René, O.; Souverneva, A.; Magnuson, S. R.; Fauber, B. P. Tetrahedron Lett. 2013, 54, 201. (c) Savoie, P. R.; Welch, J. T. Chem. Rev. 2015, 115, 1130.
(a) Bassetto, M.; Ferla, S.; Pertusati, F. Future Med. Chem. 2015, 7, 527. (b) Sowaileh, M. F.; Hazlitt, R. A.; Colby, D. A. ChemMedChem 2017, 12, 1481.
Guzyr, O. I.; Kozel, V. N.; Rusanov, E. B.; Rozhenko, A. B.; Fetyukhin, V. N.; Shermolovich, Yu. G. J. Fluorine Chem. 2020, 239, 109635.
Schmid, J. R.; Pröhm, P.; Voßnacker, P.; Thiele, G.; Ellwanger, M.; Steinhauer, S.; Riedel, S. Eur. J. Inorg. Chem. 2020, 4497.
René, O.; Souverneva, A.; Magnuson, S. R.; Fauber, B. P. Tetrahedron Lett. 2013, 54, 201.
(a) Yagupol'skii, L. M.; Gruz, B. F. J. Gen. Chem. USSR 1967, 37, 2350. (b) Pavlenko, N. V.; Zavatskii, V. N.; Semenii, V. Ya.; Matyushecheva, G. I.; Yagupol'skii, L. M. J. Gen. Chem. USSR 1989, 59, 474.
(a) Zhu, J.; Chen, Z.; Xie, H.; Li, S.; Wu, Y. Org. Lett. 2010, 12, 2434. (b) Yuan, Y.; Dong, W.; Gao, X.; Xie, X.; Zhang Z. Org. Lett. 2019, 21, 469. (c) Zhu, J.; Xie, H.; Li, S.; Chen, Z.; Wu, Y. J. Fluorine Chem. 2011, 132, 306. (d) Li, C.-L.; Zhang, X.-G.; Tang, R.-Y.; Zhong, P.; Li, J.-H. J. Org. Chem. 2010, 75, 7037.
(a) Gryshuk, A. L.; Perkins, J.; LaTour, J. V. US Patent 2011/224442. (b) Mizuno, Y.; Adachi, K.; Nakamura, K. Yakugaku Zasshi 1952, 72, 1266; Chem. Abstr. 1953, 47, 12357. (c) Yagupol'skii, L. M.; Gruz, B. E.; Katerinenko, L. I. J. Gen. Chem. USSR 1968, 38, 1688. (d) Todesco, P. E.; Vivarelli, P. Gazz. Chim. Ital. 1964, 94, 435. (e) Yoshida, M.; Hayakawa, I.; Hayashi, N.; Agatsuma, T.; Oda, Y.; Tanzawa, F.; Iwasaki, S.; Koyama, K.; Furukawa, H.; Kurakata, S.; Sugano, Y. Bioorg. Med. Chem. Lett. 2005, 15, 3328. (f) Garzón, M.; Arce, E. M.; Reddy, R. J.; Davies, P. W. Adv. Synth. Catal. 2017, 359, 1837. (g) Connolly, P. J.; Emanuel, S. L.; Huang, S.; Turchi, I. J. WO Patent 2007/121154. (h) Sondag, D.; Merx, J.; Rossing, E.; Boltje, T. J.; Löwik, D. W. P. M.; Nelissen, F. H. T.; van Geffen, M.; van't Veer, C.; van Heerde, W. L.; Rutjes, F. P. J. T. ChemBioChem 2022, 23, e202200190.
(a) Zhu, J.; Chen, Z.; Xie, H.; Li, S.; Wu, Y. Org. Lett. 2010, 12, 2434. (b) Li, C.-L.; Zhang, X.-G.; Tang, R.-Y.; Zhong, P.; Li, J.-H. J. Org. Chem. 2010, 75, 7037.
(a) Wessling, D.; Leister, H.; Degener, E. US Patent 3899504; Chem. Abstr. 1975, 77, 141492. (b) Willems, A. G. M.; Tempel, A.; Hamminga, D.; Stork, B. Recl. Trav. Chim. Pays-Bas 1971, 90, 97. (c) Nociarová, J.; Osuský, P.; Rakovský, E.; Georgiou, D.; Polyzos, I.; Fakis, M.; Hrobárik, P. Org. Lett. 2021, 23, 3460. (d) Enders, E.; Degener, E. DE Patent 1168911; Chem. Abstr. 1964, 61, 3073c.
(a) Wang, X.-Y.; Yao, X.; Narita, A.; Müllen, K. Acc. Chem. Res. 2019, 52, 2491. (b) Wang, Z.; Peng, Z.; Huang, K.; Lu, P.; Wang, Y. J. Mater. Chem. C 2019, 7, 6706.
(a) Chojnacki, K.; Wińska, P.; Karatsai, O.; Koronkiewicz, M.; Milner-Krawczyk, M.; Wielechowska, M.; Rędowicz, M. J.; Bretner, M.; Borowiecki, P. Int. J. Mol. Sci. 2021, 22, 6261. (b) Martínez, R.; Geronimo, B. D.; Pastor, M.; Zapico, J. M.; Coderch, C.; Panchuk, R.; Skorokhyd, N.; Maslyk, M.; Ramos, A.; de Pascual-Teresa, B. Molecules 2020, 25, 1497.
(a) Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3. (b) Sheldrick, G. M. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2015, C71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(4/5), 304–308
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guzyr, O.I., Potikha, L.M., Shishkina, S.V. et al. The nitration and bromination of 2-(pentafluorosulfanyl)-1,3-benzothiazole and 2-(trifluoromethyl)-1,3-benzothiazole. Chem Heterocycl Comp 59, 304–308 (2023). https://doi.org/10.1007/s10593-023-03197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03197-9