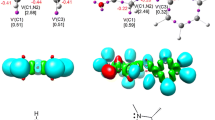
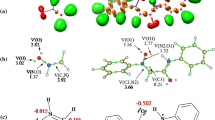
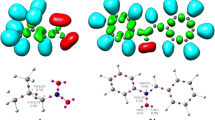
The regio- and stereoselectivity of [3+2] cycloaddition reactions of (Z)-1-(anthracen-9-yl)-N-methyl nitrone with analogs of trans-β-nitrostyrene were studied within the molecular electron density theory at the B3LYP/6-31G(d) and MPWB95/6-311G(d,p) theory levels. Analysis of the reactivity indices for presented reactions suggests that nitrone participates as nucleophile, while studied nitroalkenes play a role of electrophiles. According to electron localization function and conceptual density functional theory, kinetic and thermodynamic aspects of processes as well as analysis of all critical structures, the most favored reaction path is the formation of (3RS,4RS,5SR)-3-(anthracen-9-yl)-5-aryl-2-methyl-4-nitroisoxazolidine, independently of simulated solvent.






Similar content being viewed by others
References
Fryźlewicz, A.; Łapczuk-Krygier, A.; Kula, K.; Demchuk, O. M.; Dresler, E.; Jasiński, R. Chem. Heterocycl. Compd. 2020, 56, 120.
Lakhvich, F. A.; Koroleva, E. V.; Akhrem, A. A. Chem. Heterocycl. Compd. 1989, 25, 359.
Rescifina, A.; Chiacchio, M. A.; Corsaro, A.; De Clercq, E.; Ianuazzo, D.; Mastino, A.; Piperno, A.; Romeo, G.; Romeo, R.; Valveri, V. J. Med. Chem. 2006, 49, 709.
Mochulskaya, N. N.; Nosova, E. V.; Charushin, V. N. Chem. Heterocycl. Compd. 2021, 57, 374.
Mirzayev, F.; Viney, K.; Linh, N. N.; Gonzalez-Angulo, L.; Gegia, M.; Jaramillo, E.; Zignol, M.; Kasaeva, T. Eur. Respir. J. 2021, 57, 2003300.
Landeck, H.; Ranke, G. US Patent 5413627A.
Vretik, L.; Ritter, H. Macromolecules 2003, 36, 6340.
Sirotkina, E. V.; Efremova, M. M.; Starova, G. L.; Kuznetsov, M. A.; Molchanov, A. P. Chem. Heterocycl. Compd. 2020, 56, 1193.
Jasiński, R. Chem. Heterocycl. Compd. 2009, 45, 748.
Markitanov, Y. N.; Timoshenko, V. M. Chem. Heterocycl. Compd. 2021, 57, 1149.
Padwa, A.; Bur, S. Chem. Heterocycl. Compd. 2016, 52, 616.
Kula, K.; Dobosz, J.; Jasiński, R.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Mirosław, B.; Demchuk, O. M. J. Mol. Struct. 2020, 1203, 127473.
Xiang, J.; Zhu, T.; Dang, Q.; Bai, X. Chem. Heterocycl. Compd. 2016, 52, 601.
Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.; Jasiński, R. Molecules 2021, 26, 1364.
Martina, K.; Tagliapietra, S.; Veselov, V. V.; Cravotto, G. Front. Chem. 2019, 7, 95.
Żmigrodzka, M.; Sadowski, M.; Kras, J.; Dresler, E.; Demchuk, O. M.; Kula, K. Sci. Rad. 2022, 1, 24.
Ríos-Gutiérrez, M.; Domingo, L. R. Eur. J. Org. Chem. 2019, 2, 267.
Kula, K.; Dresler, E.; Demchuk, O. M.; Jasiński, R. Przem. Chem. 2015, 94, 1385.
Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Curr. Chem. Lett. 2019, 8, 13.
Shvekhgeimer, G. A.; Zvolinskii, V. I.; Kobrakov, K. I. Chem. Heterocycl. Compd. 1986, 22, 353.
Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiołek, Ł.; Miodowski, D.; Demchuk, O. M.; Jasiński, R. Saudi Pharm. J. 2019, 27, 593.
Domingo, L. R. Molecules 2016, 21, 1319.
Becke, A. D.; Edgecombe, K. E. J. Chem. Phys. 1990, 92, 5397.
Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. Molecules 2016, 21, 748.
Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, 1989.
Domingo, L. R.; Kula, K.; Ríos-Gutiérrez, M.; Jasiński, R. J. Org. Chem. 2021, 86, 12644.
Parr, R. G.; Szentpaly, L. V.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922.
Domingo, L. R.; Chamorro, E.; Pérez, P. J. Org. Chem. 2008, 73, 4615.
Domingo, L. R.; Ríos-Gutiérrez, M. In Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory; Liu, S., Ed.; WILEY-VCH GmbH: Weinheim, 2022, vol. 2, p. 481.
Aurell, M. J.; Domingo, L. R.; Pérez, P.; Contreras, R. Tetrahedron 2004, 60, 11503.
Domingo, L. R.; Pérez, P.; Sáez, J. A. RSC Adv. 2013, 3, 1486.
Benchouk, W.; Mekelleche, S. M.; Silvi, B.; Aurell, M. J.; Domingo, L. R. J. Phys. Org. Chem. 2011, 24, 611.
Jasiński, R. Chem. Heterocycl. Compd. 2022, 58, 260.
Domingo, L. R.; Ríos-Gutiérrez, M. Org. Biomol. Chem. 2019, 17, 6478.
Domingo, L. R. RSC Adv. 2014, 4, 32415.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, 2013.
Zhao, Y.; Truhlar, G. D. J. Phys. Chem. A 2004, 108, 6908.
Zawadzińska, K.; Ríos-Gutiérrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. Molecules 2021, 26, 6774.
Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Pure Appl. Chem. 2021, 93, 427.
Kula, K.; Łapczuk-Krygier, A. Curr. Chem. Lett. 2018, 7, 27.
Demchuk, O. M.; Jasinski, R.; Strzelecka, D.; Dziuba, K.; Kula, K.; Chrzanowski, J.; Krasowska, D. Pure Appl. Chem. 2018, 90, 49.
Fukui, K. J. Phys. Chem. 1970, 74, 4161.
Tapia, O. J. Math. Chem. 1992, 10, 131.
Tomasi, J.; Perisco, M. Chem. Rev. 1994, 94, 2027.
Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Chem. Phys. Lett. 1996, 225, 327.
Mlostoń, G.; Jasiński, R.; Kula, K.; Heimgartner, H. Eur. J. Org. Chem. 2020, 2, 176.
Kula, K.; Zawadzińska, K. Curr. Chem. Lett. 2021, 10, 9.
Mlostoń, G.; Kula, K.; Jasiński, R. Molecules 2021, 26, 5562.
Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Comput. Chem. 1999, 23, 597.
Dennington, R.; Keith, T. A.; Millam, J. M. GaussView, Version 6.; Semichem, Inc.: Shawnee Mission, 2016.
Ahrens, J.; Geveci, B.; Law, C. In ParaView: An End-User Tool for Larga Data Visualization. The Visualization Handbook; Elsevier: Amsterdam, 2005.
Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware: New York, 2015.
This work was partially supported by PLGrid Infrastructure.
All calculations reported in this paper were performed on Prometheus supercomputer cluster in the CYFRONET computational center in Cracow.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(3), 138–144
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kula, K., Sadowski, M. Regio- and stereoselectivity of [3+2] cycloaddition reactions between (Z)-1-(anthracen-9-yl)-N-methyl nitrone and analogs of trans-β-nitrostyrene on the basis of MEDT computational study. Chem Heterocycl Comp 59, 138–144 (2023). https://doi.org/10.1007/s10593-023-03175-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03175-1