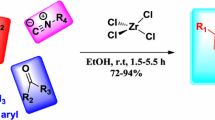
A method for the introduction of the N,N-dimethylformamidine protective group in a series of six-membered 2-aminohetarene N-oxides was developed. It was shown that 2-amino derivatives of N-oxides of various six-membered heterocycles (pyridines, pyrimidines, pyrazines, tetrazines) successfully enter the reaction. The stability of the N,N-dimethylformamidine protecting group in 2-aminohetarene N-oxides was found to depend on the type of reaction medium.




Similar content being viewed by others
References
Makhova, N. N.; Belen'kii, L. I.; Gazieva, G. A.; Dalinger, I. L.; Konstantinova, L. S.; Kuznetsov, V. V.; Kravchenko, A. N.; Krayushkin, M. M.; Rakitin, O. A.; Starosotnikov, A. M.; Fershtat, L. L.; Shevelev, S. A.; Shirinian, V. Z.; Yarovenko, V. N. Russ. Chem. Rev. 2020, 89, 55. [Usp. Khim. 2020, 89, 55.]
(a) Zlotin, S. G.; Dalinger, I. L.; Makhova, N. N.; Tartakovsky, V. A. Russ. Chem. Rev. 2020, 89, 1. [Usp. Khim. 2020, 89, 1.] (b) Gulyaev, D. A.; Klenov, M. S.; Churakov, A. M.; Strelenko, Yu. A.; Pivkina, A. N.; Tartakovsky, V. A. Russ. Chem. Bull., Int. Ed. 2021, 70, 1599. [Izv. Akad. Nauk, Ser. Khim. 2021, 1599.] (c) Zlotin, S. G.; Churakov, A. M.; Egorov, M. P.; Fershtat, L. L.; Klenov, M. S.; Kuchurov, I. V.; Makhova, N. N.; Smirnov, G. A.; Tomilov, Yu. V.; Tartakovsky, V. A. Mendeleev Commun. 2021, 31, 731.
(a) Zyryanov, G. V.; Kopchuk, D. S.; Kovalev, I. S.; Santra, S.; Rahman, M.; Khasanov, A. F.; Krinochkin, A. P.; Taniya, O. S.; Chupakhin, O. N.; Charushin, V. N. Mendeleev Commun. 2020, 30, 537. (b) Sedenkova, K. N.; Nazarova, A. A.; Zverev, D. V.; Zharmuhambetova, Zh. T.; Vasilenko, D. A.; Grishin, Yu. K.; Kuznetsova, T. S.; Averina, E. B. Russ. Chem. Bull., Int. Ed. 2021, 70, 1311. [Izv. Akad. Nauk, Ser. Khim. 2021, 1311.]
Volkova, Yu. M.; Makarov, A. Yu.; Pritchina, E. A.; Gritsan, N. P.; Zibarev, A. V. Mendeleev Commun. 2020, 30, 385.
Das, P.; Delost, M. D.; Qureshi, M. H.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2019, 62, 4265.
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
Koukal, P.; Ulč, J.; Nečas, D.; Kotora, M. Top. Heterocycl. Chem. 2017, 53, 29.
Mfuh, A. M.; Larionov, O. V. Curr. Med. Chem. 2015, 22, 2819.
Stephens, D. E.; Larionov, O. V. Top. Heterocycl. Chem. 2017, 53, 59.
Kutasevich, A. V.; Perevalov, V. P.; Mityanov, V. S. Eur. J. Org. Chem. 2021, 357.
Bystrov, D. M.; Zhilin, E. S.; Fershtat, L. L.; Romanova, A. A.; Ananyev, I. V.; Makhova, N. N. Adv. Synth. Catal. 2018, 360, 3157.
Brown, E. V. J. Am. Chem. Soc. 1957, 79, 3565.
Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, Z.-J.; Zheng, X.-X.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Angew. Chem., Int. Ed. 2015, 54, 272.
Krasnokutskaya, E. A.; Chudinov, A. A.; Filimonov, V. D. Synthesis 2018, 1368.
Kidjemet, D. Synlett 2002, 1741.
Gümüş, M.; Koca, İ. ChemistrySelect 2020, 5, 12377.
Wolińska, E.; Pucko, W. J. Heterocycl. Chem. 2013, 50, 590.
Wolińska, E. Heterocycl. Commun. 2012, 18, 227.
Wolińska, E. Heterocycl. Commun. 2016, 22, 85.
Hovhannisyan, A.; Pham, T. H.; Bouvier, D.; Piroyan, A.; Dufau, L.; Qin, L.; Cheng, Y.; Melikyan, G.; Reboud-Ravaux, M.; Bouvier-Durand, M. Bioorg. Med. Chem. Lett. 2014, 24, 1571.
Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C. Acta Crystallogr., Sect. B 2016, 72, 171.
Deady, L. W. Synth. Commun. 1977, 7, 509.
Wei, H.; Gao, H.; Shreeve, J. M. Chem.–Eur. J. 2014, 20, 16943.
Coburn, M. D.; Hiskey, M. A.; Lee, K.-Y.; Ott, D. G.; Stinecipher, M. M. J. Heterocycl. Chem. 1993, 30, 1593.
Bruker APEX-III; Bruker AXS, Inc.: Madison, 2018.
Krause, L.; Herbst-Irmer, R.; Sheldrick, G. M.; Stalke, D. J. Appl. Crystallogr. 2015, 48, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, 2016.
Perdew, J. P.; Ernzerhof, M.; Burke, K. J. Chem. Phys. 1996, 105, 9982.
Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158.
Grimme, S.; Ehrlich, S.; Goerigk, L. J. Comput. Chem. 2011, 32, 1456.
This work was carried out with the financial support of the Russian Foundation for Basic Research and the Moscow Government within the framework of the scientific project No. 21-33-70056.
X-ray structural studies and quantum-chemical calculations were carried out with the support of the Ministry of Science and Higher Education of the Russian Federation using the scientific equipment of the Center for Molecular Structure Studies of A. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(11), 1130–1136
Supplementary Information
ESM 1
(PDF 1841 kb)
Rights and permissions
About this article
Cite this article
Karalash, S.А., Bystrov, D.M., Ananyev, I.V. et al. Lewis acid catalyzed condensation of 2-aminohetarene N-oxides with N,N-dimethylformamide dimethyl acetal. Chem Heterocycl Comp 57, 1130–1136 (2021). https://doi.org/10.1007/s10593-021-03031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-03031-0