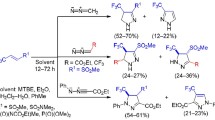
Abstract
The influence of the structures of N-alkyl-3-aminopyrazoles on their transformation into azopyrazoles on treatment with sodium hypohalogenites was studied. The reaction of 3-aminopyrazoles unsubstituted at position 4 containing donor substituents with neutral solutions of sodium hypohalogenites leads to mixtures of 3,3′-azopyrazoles (yields 1–40%) and 4,4′-dihalo-3,3′-azopyrazoles (yields 20–79%). In this case, generation of 3,3′-azopyrazoles is favored by the addition of NaOH to the reaction mixture. The N—N coupling of aminopyrazoles with acceptor substituents in the aromatic ring results in the selective formation of 3,3′-azopyrazoles even in neutral media. The reactions of 4-substituted 3-aminopyrazoles with NaOBr afford only 3,3′-azopyrazoles. The regularities of occurrence of all the above processes are discussed.
Similar content being viewed by others
References
B. V. Lyalin, V. L. Sigacheva, V. A. Kokorekin, V. A. Petrosyan, Mendeleev Commun., 2015, 25, 479.
B. V. Lyalin, V. L. Sigacheva, V. A. Kokorekin, V. A. Petrosyan, Arkivoc, 2017, Part iii, 3, 55.
B. V. Lyalin, V. L. Sigacheva, V. A. Kokorekin, V. A. Petrosyan, Tetrahedron Lett., 2018, 59, 2741.
Yu. Yu. Lur’e, Spravochnik po analiticheskoi khimii [Handbook on Analytical Chemistry], Khimiya, Moscow, 1965, 217 pp. (in Russian).
J. Catalán, M. Menéndez, J. Laynez, R. M. Claramunt, M. Bruix, J. De Mendoza, J. Elguero, J. Heterocycl. Chem., 1985, 22, 997.
B. V. Lyalin, V. A. Petrosyan, B. I. Ugrak, Russ. J. Electrochem., 2008, 44, 1320.
R. Zawalski, P. Kovacic, J. Org. Chem, 1979, 44, 2130.
I. M. Kolthoff, R. Belcher, V. A. Stenger, G. Matsuyama, Volumetric Analysis, Vol. 3, Intersci. Publ., New York—London, 1957, 714 pp.
B. V. Lyalin, V. L. Sigacheva, V. A. Kokorekin, T. Yu. Dutova, G. M. Rodionova, V. A. Petrosyan, Russ. Chem. Bull., 2018, 67, 510.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 164–170, January, 2021.
Rights and permissions
About this article
Cite this article
Lyalin, B.V., Sigacheva, V.L., Ugrak, B.I. et al. Oxidative N—N coupling of N-alkyl-3-aminopyrazoles to azopyrazoles in aqueous solutions of NaOCl and NaOBr. Russ Chem Bull 70, 164–170 (2021). https://doi.org/10.1007/s11172-021-3072-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3072-z