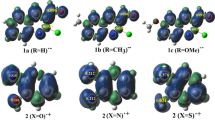
N,O-Diprotonated forms (dications) of various quinolinecarbaldehydes were theoretically studied by DFT calculations. It was found that the most reactive electrophilic dications are expected to be generated from 2- and 4-quinolinecarbaldehydes compared to the other quinolinecarbaldehydes. Experimental studies of protonation of quinoline-2(6,8)-carbaldehydes in Brønsted acids (CF3SO3H, H2SO4) by means of 1H, 13C, and 15N NMR revealed the formation of the corresponding N-protonated O-protosolvated species. Reactions of quinoline-2(6,8)-carbaldehydes with arenes in the presence of Brønsted (TfOH) and Lewis acids (AlX3, X = Cl, Br) or acidic zeolites led to the formation of the corresponding 2(6,8)-(diarylmethyl)quinolines. However, 6- and 8-quinolinecarbaldehydes gave additionally unusual products – 6(8)-(arylmethyl)quinolines.



Similar content being viewed by others
References
Ebenso, E. E.; Obot, I. B.; Murulana, L. C. Int. J. Electrochem. Sci. 2010, 5, 1574.
Mohan, V.; Das, N.; Jain, V. K.; Khan, T.; Pandey, S. K.; Faizi, Md. S. H.; Daniel, J.; Sen, P. ChemistrySelect 2020, 5, 9435.
Romanović, M. Č.; Čobeljić, B.; Pevec, A.; Turel, I.; Anđelković, K.; Milenković, M.; Radanović, D.; Belošević, S.; Milenković, M. R. J. Coord. Chem. 2017, 70, 2425.
Bjelogrlić, S. K.; Todorović, T. R.; Kojić, M.; Senćanski, M.; Nikolić, M.; Višnjevac, A.; Araškov, J.; Miljković, M.; Muller, C. D.; Filipović, N. R. J. Inorg. Biochem. 2019, 199, 110758.
Liang, F.; Xie, Z.; Wang, L.; Jing, X.; Wang, F. Tetrahedron Lett. 2002, 43, 3427.
Korivi, R. P.; Cheng, C.-H. J. Org. Chem. 2006, 71, 7079.
Kim, J. I.; Shin, I.-S.; Kim, H.; Lee, J.-K. J. Am. Chem. Soc. 2005, 127, 1614.
Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Chem. Soc. Rev. 2021, 50, 1030.
Li, W.-Y.; Miao, K.; Wu, H.-L.; He, X.-W.; Liang, H. Microchim. Acta 2003, 143, 33.
Zhang, N.; Zhang, H.-S.; Wang, H. Electrophoresis 2009, 30, 2258.
Damant, A. P. In Handbook of Textile and Industrial Dyeing; Woodhead Publishing, 2011, vol. 2, p. 252.
Upadhyay, K. D.; Dodia, N. M.; Khunt, R. C.; Chaniara, R. S.; Shah, A. K. ACS Med. Chem. Lett. 2018, 9, 283.
Sharma, P. C.; Chaudhary, M.; Sharma, A.; Piplani, M.; Rajak, H.; Prakash, O. Curr. Top. Med. Chem. 2013, 13, 2076.
Zhang, Z.; Xiao, X.; Su, T.; Wu, J.; Ren, J.; Zhu, J.; Zhang, X.; Cao, R.; Du, R. Eur. J. Med. Chem. 2017, 140, 239.
Fan, Y.-L.; Wu, J.-B.; Cheng, X.-W.; Zhang, F.-Z.; Feng, L.-S. Eur. J. Med. Chem. 2018, 146, 554.
Zhang, S.; Xu, Z.; Gao, C.; Ren, Q.-C.; Chang, L.; Lv, Z.-S.; Feng. L.-S. Eur. J. Med. Chem. 2017, 138, 501.
Xu, Z.; Zhang, S.; Gao, C.; Fan, J.; Zhao, F.; Lv, Z.-S.; Feng, L.-S. Chin. Chem. Lett. 2017, 2, 159.
Cannalire, R.; Barreca, M. L.; Manfroni, G.; Cecchetti, V. J. Med. Chem. 2016, 59, 16.
Zhang, G.-F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.-L. Eur. J. Med. Chem. 2018, 146, 599.
Zhang, G.-F.; Zhang, S.; Pan, B.; Liu, X.; Feng, L.-S. Eur. J. Med. Chem. 2018, 143, 710.
Wang, Z.; Hu, J.; Yang, X.; Feng, X.; Li, X.; Huang, L.; Chan, A. S. C. J. Med. Chem. 2018, 61, 1871.
Sekgota, K. C.; Majumder, S.; Isaacs, M.; Mnkandhla, D.; Hoppe, H. C.; Khanye, S. D.; Kriel, F. H.; Coates, J.; Kaye, P. T. Bioorg. Chem. 2017, 75, 310.
Luo, Z. G.; Tan, J. J.; Zeng, Y.; Wang, C. X.; Hu, L. M. Mini Rev. Med. Chem. 2010, 10, 1046.
Achan, J.; Talisuna, A. O.; Erhart, A.; Yeka, A.; Tibenderana, J. K.; Baliraine, F. N.; Rosenthal, P. J.; D'Alessandro, U. Malar. J. 2011, 10, 144.
Kouznetsov, V. V.; Vargas Méndez, L. Y.; Meléndez Gómez, C. M. Cur. Org. Chem. 2005, 9, 141.
Ryabukhin, D. S.; Vasilyev, A. V. Russ. Chem. Rev. 2016, 85, 637. [Usp. Khim. 2016, 85, 637. ]
Khusnutdinov, R. I.; Bayguzina, A. R.; Dzhemilev, U. M. J. Organomet. Chem. 2014, 768, 75.
Madapa, S.; Tusi, Z.; Batra, S. Curr. Org. Chem. 2008, 12, 1116.
Boyarskiy, V. P.; Ryabukhin, D. S.; Bokach, N. A.; Vasilyev, A. V. Chem. Rev. 2016, 116, 5894.
Koltunov, K. Yu.; Prakash, G. K. S.; Rasul, G.; Olah, G. A. J. Org. Chem. 2002, 67, 4330.
Klumpp, D. A.; Jones, A.; Lau, S.; de Leon, S.; Garza, M. Synthesis 2000, 1117.
Prakash, G. K. S.; Paknia, F.; Chacko, S.; Mathew, T.; Olah, G. A. Heterocycles 2008, 76, 783.
Gurskaya, L. Yu.; Belyanskaya, D. S.; Ryabukhin, D. S.; Nilov, D. I.; Boyarskaya, I. A.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 950.
Ryabukhin, D. S.; Zakusilo, D. N.; Kompanets, M. O.; Tarakanov, A. A.; Boyarskaya, I. A.; Artamonova, T. O.; Khohodorkovskiy, M. A.; Opeida, I. O.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 2125.
Ryabukhin, D. S.; Turdakov, A. N.; Soldatova, N. S.; Kompanets, M. O.; Ivanov, A. Yu.; Boyarskaya, I. A.; Vasilyev, A. V. Beilstein. J. Org. Chem. 2019, 15, 1962.
Borisova, M. A.; Ryabukhin, D. S.; Vasilyev, A. V. Chem. Heterocycl. Compd. 2020, 56, 964.
Parr, R. G.; Szentpály, L. V.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922.
Pelt, V. P.; Buck, H. M. J. Am. Chem. Soc. 1976, 98, 5864.
Bartlett, P. D.; McCollu, J. D. J. Am. Chem. Soc. 1956, 78, 1441.
Gronert, S.; Keeffe, J. R. J. Am. Chem. Soc. 2005, 127, 2324.
Pelt, van, P. Proton Acid Catalysed Hydride Transfer from Alkanes to Methylated Benzyl Cations; Technische Hogeschool Eindhoven: Eindhoven, 1975.
Roberts, R. M.; El-Khawaga, A. M., Sweeney. K. M.; El-Zohry, M. F. J. Org. Chem. 1987, 52, 1591.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J.; Gaussian 09, Revision C.01, Gaussian, Inc.: Wallingford, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(10), 1007–1016
Supplementary Information
ESM 1
(PDF 3165 kb)
Rights and permissions
About this article
Cite this article
Borisova, M.А., Ryabukhin, D.S., Ivanov, A.Y. et al. Reactions of Quinolinecarbaldehydes with Arenes under Superelectrophilic Activation. NMR and DFT Studies of Dicationic Electrophilic Species. Chem Heterocycl Comp 57, 1007–1016 (2021). https://doi.org/10.1007/s10593-021-03015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-03015-0