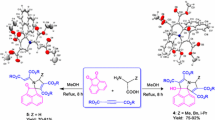
A regio- and stereoselective method for the synthesis of spiro[acenaphthylenepyrroli(zi)din]-2-ones containing a 1,3-diketone fragment in their structure in 57–93% yields was developed. The method is based on (3+2) cycloaddition of azomethine ylides generated in situ from acenaphthenequinone and α-amino acids (L-(thia)proline, L-phenylglycine) to (E)-1,5-diarylpent-4-ene-1,3-diones in EtOH at 60–70°C for 4–8 h.


Similar content being viewed by others
References
Kumar, R. S.; Antonisamy, P.; Almansour, A. I.; Arumugam, N.; Al-thamili, D. M.; Kumar, R. R.; Kim, H.-R.; Kwon, K.-B. Bioorg. Chem. 2019, 91, 103180.
Wei, A. C.; Ali, M. A.; Yoon, Y. K.; Ismail, R.; Choon, T. S.; Kumar, R. S. Bioorg. Med. Chem. Lett. 2013, 23, 1383.
Dandia, A.; Kumari, S.; Soni, P. Eur. Chem. Bull. 2013, 2, 1004.
Periyasami, G.; Arumugam, N.; Rahaman, M.; Kumar, R. S.; Manikandan, M.; Alfayez, M. A.; Premnath, D.; Aldalbahi, A. RSC Adv. 2018, 8, 16303.
(a) Döndas, H. A.; de Gracia Retamosa, M.; Sansano, J. M. Synthesis 2017, 2819. (b) Arumugam, N.; Kumar, R. S.; Almansour, A. I.; Perumal, S. Curr. Org. Chem. 2013, 17, 1929. (c) Najera, C.; Sansano, J. M. Pure Appl. Chem. 2019, 91, 575. (d) Izmest'ev, A. N.; Gazieva, G. А.; Kravchenko, A. N. Chem. Heterocycl. Compd. 2020, 56, 255. [Khim. Geterotsikl. Soedin. 2020, 56, 255.] (e) Molteni, G.; Silvani, A. Eur. J. Org. Chem. 2021, 1653. e Singh, R.; Bhardwaj, D.; Saini, M. R. RSC Adv. 2021, 11, 4760. f Korotaev, V. Yu.; Zimnitskiy, N. S.; Barkov, A. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2018, 54, 905. [Khim. Geterotsikl. Soedin. 2018, 54, 905.] (h) Xiong, Y.; Han, X.-X.; Lu, Y.; Wang, H.-J.; Zhang, M.; Liu, X.-W. Tetrahedron 2021, 87, 132112. g Ling, Y.; Huang, Y.; Li, X. Chem. Heterocycl. Compd. 2021, 57, 181. [Khim. Geterotsikl. Soedin. 2021, 57, 181.] (j) Chithiraikumar, C.; Ponmuthu, K. V.; Harikrishnan, M.; Malini, N.; Sepperumal, M.; Siva, A. Res. Chem. Intermed. 2021, 47, 895. h Pavlovska, T. L.; Lipson, V. V.; Shishkina, S. V.; Musatov, V. I.; Borisov, A. V.; Mazepa, A. V. Chem. Heterocycl. Compd. 2019, 55, 679. [Khim. Geterotsikl. Soedin. 2019, 55, 679.] (l) Filatov, A. S.; Knyazev, N. A.; Molchanov, A. P.; Panikorovsky, T. L.; Kostikov, R. R.; Larina, A. G.; Boitsov, V. M.; Stepakov, A. V. J. Org. Chem. 2017, 82, 959. i Barkov, A. Yu.; Zimnitskiy, N. S.; Kutyashev, I. B.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 1315. [Khim. Geterotsikl. Soedin. 2017, 53, 1315.] (n) Klochkova, I. N.; Shchekina, M. P.; Anis'kov, A. A. Chem. Heterocycl. Compd. 2014, 50, 479. [Khim. Geterotsikl. Soedin. 2014, 527.]
(a) Kumar, R. S.; Almansour, A. I.; Arumugam, N.; Periyasami, G.; Athimoolam, S.; Kumar, R. R.; Asad, M.; Asiri, A. M. Tetrahedron Lett. 2018, 59, 3336. (b) Yavari, I.; Baoosi, L.; Halvagar, M. R. Synlett 2018, 635. (c) Kutyashev, I. B.; Sannikov M. S.; Kochnev, I. A.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. SynOpen 2021, 5, 1. (d) Maurya, R. A.; Nayak, R.; Reddy, C. N.; Kapure, J. S.; Nanubolu, J. B.; Singarapu, K. K.; Ajitha, M.; Kamal, A. RSC Adv. 2014, 4, 32303. (e) Boudriga, S.; Haddad, S.; Murugaiyah, V.; Askri, M.; Knorr, M.; Strohmann, C.; Golz, C. Molecules 2020, 25, 1963. (f) Shahrestani, N.; Khosravi, H.; Jadidi, K.; Notash, B.; Naderi, S. Org. Biomol. Chem. 2019, 17, 7013. (g) Purushothaman, S.; Prasanna, R.; Raghunathan, R. Tetrahedron 2013, 69, 9742. (h) Shi, C.; Zhou, J. Heterocycles 2015, 91, 1972. (i) Rouatbi, F.; Askri, M.; Nana, F.; Kirsch, G.; Sriram, D.; Yogeeswari, P. Tetrahedron Lett. 2016, 57, 163. (j) Thimmarayaperumal, S.; Shanmugam, S. New J. Chem. 2018, 42, 4061. (k) Dandia, A.; Parewa, V.; Kumari, S.; Bansal, S.; Sharma, A. Green Chem. 2016, 18, 2488. (l) Sivakumar, S.; Kumar, R. R.; Ali, M. A.; Choon, T. S. Eur. J. Med. Chem. 2013, 65, 240. (m) Sumesh, R. V.; Shylaja, A.; Kumar, R. R.; Almansour, A. I.; Kumar, R. S. Tetrahedron Lett. 2018, 59, 4086.
(a) Arrieta, A.; Beyer, L.; Kleinpeter, E.; Lehmann, J.; Dargatz, M. J. Prakt. Chem. 1992, 334, 696. (b) Jang, Y.-J.; Chen, Y.-S.; Lee, C.-J.; Chen, C.-H.; Lin, W. Synthesis 2015, 95. (c) Pinto, J.; Silva, V. L. M.; Silva, A. M. G.; Silva, A. M. S. Molecules 2015, 20, 11418. (d) Liou, Y.-C.; Su, Y.-H.; Ku, K.-C.; Edukondalu, A.; Lin, C.-K.; Ke, Y.-S.; Karanam, P.; Lee, C.-J.; Lin, W. Org. Lett. 2019, 21, 8339. (e) Thomas, A. R.; Shuler, W. G.; Smith, E. A.; Carlisle, S. S.; Knick, S. L.; Puciaty, A. J.; Metz, C. R.; VanDerveer, D. G.; McMillen, C. D.; Pennington, W. T.; Beam, C. F. J. Chem. Crystallogr. 2013, 43, 629.
(a) Zimnitskiy, N. S.; Barkov, A. Yu.; Ulitko, M. V.; Kutyashev, I. B.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. New J. Chem. 2020, 44, 16185. (b) Korotaev, V. Yu.; Zimnitskiy, N. S.; Denikaev, A. D.; Barkov, A. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 81. [Khim. Geterotsikl. Soedin. 2021, 57, 81.]
Zimnitskiy, N. S.; Denikaev, A. D.; Barkov, A. Yu.; Kutyashev, I. B.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. J. Org. Chem. 2020, 85, 8683.
(a) Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112. (b) Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(7/8), 743–750
Supplementary Information
ESM 1
(PDF 2334 kb)
Rights and permissions
About this article
Cite this article
Zimnitskiy, N.S., Barkov, A.Y., Kutyashev, I.B. et al. Acenaphthenequinone-Based Stabilized Azomethine Ylides in (3+2) Cycloaddition Reactions with 1,5-diarylpent-4-ene-1,3-diones. Chem Heterocycl Comp 57, 743–750 (2021). https://doi.org/10.1007/s10593-021-02978-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02978-4