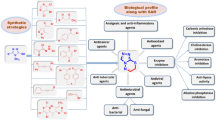
This review compiles literature data on the design of biologically active compounds based on 2-aminopyrimidin-4(3H)-one and its derivatives over the past 20 years. The systematization and analysis of the collected data made it possible to identify the priority directions of this process, including the development of antiviral and antileukemic agents, drugs for combating hyperuricemia, neurodegenerative disorders, some forms of cancer and, finally, anti-inflammatory and hormonal drugs.






Similar content being viewed by others
References
(a) Helene, C.; Douzou, P. Compt. Rend. 1964, 259, 4387. (b) Brown, D. J.; Teitei, T. Aust. J. Chem. 1965, 18, 559.
(a) Sharma, B. D.; McConnell, J. F. Acta Crystallogr. 1965, 19, 797. (b) Portalone, G.; Colapietro, M. Acta Crystallogr., Sect. E.: Crystallogr. Commun. 2007, 63, o1869.
Wierenga, W.; Skulnick, H. I.; Stringfellow, D. A.; Weed, S. D.; Renis, H. E.; Eidson, E. E. J. Med. Chem. 1980, 23, 237.
Lotzova, E.; Savary, C. A.; Khan, A.; Stringfellow, D. A. J. Immunol. 1984, 132, 2566.
(a) Koroleva, E. V.; Gusak, K. N.; Ignatovich, Zh. V. Russ. Chem. Rev. 2010, 79, 655. [Usp. Khim. 2010, 79, 720.] (b) Goel, R.; Luxami, V.; Paul, K. RSC Adv. 2015, 5, 81608. a Dolzhenko, A. V. Heterocycles 2011, 83, 1489.
Sharma, V.; Chitranshi, N.; Agarwal, A. K. Int. J. Med. Chem. 2014, 202784. DOI: https://doi.org/10.1155/2014/202784.
Verbitskiy, E. V.; Rusinov, G. L.; Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Bull., Int. Ed. 2019, 68, 2172. [Izv. Akad. Nauk, Ser. Khim. 2019, 2172.]
Novakov, I. A.; Navrotskii, M. B.; Zakharova, E. K.; Brunilina, L. L. Russ. Chem. Bull., Int. Ed. 2015, 64, 2545. [Izv. Akad. Nauk, Ser. Khim. 2015, 2545.].
(a) Mai, A.; Artico, M.; Rotili, D.; Tarantino, D.; Clotet- Codina, I.; Armand-Ugon, M.; Ragno, R.; Simeoni, S.; Sbardella, G.; Nawrozkij, M. B.; Samuele, A.; Maga, G.; Esté, J. A. J. Med. Chem. 2007, 50, 5412. (b) Navrotskii, M. B. Extended Abstract of Doctoral (Chem.) Thesis, Moscow, 2012.
Holý, A.; Günter, J.; Dvořáková, H.; Masojídková, M.; Andrei, G.; Snoeck, R.; Balzarini, J.; De Clercq, E. J. Med. Chem. 1999, 42, 2064.
(a) Holý, A.; Votruba, I.; Masojídková, M.; Andrei, G.; Snoeck, R.; Naesens, L.; De Clercq, E.; Balzarini, J. J. Med. Chem. 2002, 45, 1918. (b) De Clercq, E.; Desire, A.; Holy, A.; Balzarini, J. AU Patent 2002315625.
Custelcean, R.; Craciun, L. Tetrahedron 2000, 56, 5067.
De Clercq, E. Antiviral. Res. 2002, 55, 1.
Abdel-Rahman, A. A. H. J. Chem. Res. 2007, 332.
Burchenal, J. H.; Ciovacco, K.; Kalaher, K.; O'Toole, T.; Kietner, R.; Dowling, M. D.; Chu, C. K.; Watanabe, K. A.; Wempen, I.; Fox, J. J. Cancer Res. 1976, 36, 1520.
Woodcock, T. M.; Chou, T.-C.; Tan, C. T. C.; Sternberg, S. S.; Philips, F. S.; Young, C. W.; Burchenal, J. H. Cancer Res. 1980, 40, 4243.
Serpi, M.; De Biasi, R.; Pertusati, F.; Slularczuk, M.; McGuigan, C. ChemistryOpen 2017, 6, 424.
Wellington, K. W.; Ooi, H. C.; Benner, S. A. Nucleosides, Nucleotides Nucleic Acids 2009, 28, 275.
Mayer, A.; Häberli, A.; Leumann, C. J. Org. Biomol. Chem. 2005, 3, 1653.
Doi, Y.; Chiba, J.; Morikawa, T.; Inouye, M. J. Am. Chem. Soc. 2008, 130, 8762.
Chu, C. K.; Wempen, I.; Watanabe, K. A.; Fox, J. J. J. Org. Chem. 1976, 41, 2793.
Bilbao, N.; Vázquez-González, V.; Teresa Aranda, M.; González-Rodríguez, D. Eur. J. Org. Chem. 2015, 7160.
Fox, K. R. Curr. Med. Chem. 2000, 7, 17.
(a) Promel, R.; Cardon, A.; Daniel, M.; Jacques, G.; Vandersmissen, A. Tetrahedron Lett. 1968, 9, 3067. (b) Hirota, K.; Yamada, Y.; Kitade, Y.; Senda, S. J. Chem. Soc., Perkin Trans. 1 1981, 2943.
Filichev, V. V.; Pedersen, E. B. Tetrahedron 2001, 57, 9163.
Liu, L. J.; Hong, J. H. Nucleosides, Nucleotides Nucleic Acids 2010, 29, 257.
Liu, J.; Liu, W.; Zhang, Z.; Cheng, Z.; Ma, X.; Wang, X.; Liu, J. Synth. Commun. 2009, 39, 2244.
Singh, K.; Singh, K.; Balzarini, J. Eur. J. Med. Chem. 2013, 67, 428.
Xu, C.; Liu, S.; Song, F.; Liu, Z. Curr. Tradit. Med. 2015, 1, 41.
Patel, A.; Lewis, W.; Searle, M. S.; Stevens, M. F. G.; Moody, C. J. Tetrahedron 2015, 71, 7339.
B-Rao, C.; Kulkarni-Almeida, A.; Katkar, K. V.; Khanna, S.; Ghosh, U.; Keche, A.; Shah, P.; Srivastava, A.; Korde, V.; Nemmani, K. S. V.; Deshmukh, N. J.; Dixit, A.; Brahma, M. K.; Bahirat, U.; Doshi, L.; Sharma, R.; Sivaramakrishnan, H. Bioorg. Med. Chem. 2012, 20, 2930.
Khanna, S.; Burudkar, S.; Bajaj, K.; Shah, P.; Keche, A.; Ghosh, U.; Desai, A.; Srivastava, A.; Kulkarni-Almeida, A.; Deshmukh, N. J.; Dixit, A.; Brahma, M. K.; Bahirat, U.; Doshi, L.; Nemmani, K. V. S.; Tannu, P.; Damre, A.; B-Rao, C.; Sharma, R.; Sivaramakrishnan, H. Bioorg. Med. Chem. Lett. 2012, 22, 7543.
Bajaj, K.; Burudkar, S.; Shah, P.; Keche, A.; Ghosh, U.; Tannu, P.; Khanna, S.; Srivastava, A.; Deshmukh, N. J.; Dixit, A.; Ahire, Y.; Damre, A.; Nemmani, K. S. V.; Kulkarni-Almeida, A.; B-Rao, C.; Sharma, R.; Sivaramakrishnan, H. Bioorg. Med. Chem. Lett. 2013, 23, 834.
Taylor, E. C.; Young, W. B. J. Org. Chem. 1995, 60, 7947.
Rodrigues, M. V. N.; Barbosa, A. F.; da Silva, J. F.; dos Santos, D. A.; Vanzolini, K. L.; de Moraes, M. C.; Corrêa, A. G.; Cass, Q. B. Bioorg. Med. Chem. 2016, 24, 226.
Huang, W.-H.; Sheng, R.; Hu, Y.-Z. Curr. Med. Chem. 2009, 16, 1806.
Edwards, P. D.; Albert, J. S.; Sylvester, M.; Aharony, D.; Andisik, D.; Callaghan, O.; Campbell, J. B.; Carr, R. A.; Chessari, G.; Congreve, M.; Frederickson, M.; Folmer, R. H. A.; Geschwindner, S.; Koether, G.; Kolmodin, K.; Krumrine, J.; Mauger, R. C.; Murray, C. W.; Olsson, L.-L.; Patel, S.; Spear, N.; Tian, G. J. Med. Chem. 2007, 50, 5912.
Yonezawa, S.; Yamamoto, T.; Yamakawa, H.; Muto, C.; Hosono, M.; Hattori, K.; Higashino, K.; Yutsudo, T.; Iwamoto, H.; Kondo, Y.; Sakagami, M.; Togame, H.; Tanaka, Y.; Nakano, T.; Takemoto, H.; Arisawa, M.; Shuto, S. J. Med. Chem. 2012, 55, 8838.
Yonezawa, S.; Yamakawa, H.; Muto, C.; Hosono, M.; Yamamoto, T.; Hattori, K.; Sakagami, M.; Togame, H.; Tanaka, Y.; Nakano, T.; Takemoto, H.; Arisawa, M.; Shuto, S. Bioorg. Med. Chem. Lett. 2013, 23, 2912.
Yonezawa, S.; Fujiwara, K.; Yamamoto, T.; Hattori, K.; Yamakawa, H.; Muto, C.; Hosono, M.; Tanaka, Y.; Nakano, T.; Takemoto, H.; Arisawa, M.; Shuto, S. Bioorg. Med. Chem. 2013, 21, 6506.
Pauletti, P. M.; Cintra, L. S.; Braguine, C. G.; da Silva Filho, A. A.; e Silva, M. L. A.; Cunha, W. R.; Januário, A. H. Mar. Drugs. 2010, 8, 1526.
(a) Tibiletti, F.; Simonetti, M.; Nicholas, K. M.; Palmisano, G.; Parravicini, M.; Imbesi, F.; Tollari, S.; Penoni, A. Tetrahedron 2010, 66, 1280. (b) Cheng, G.; Cushing, T. D.; Fisher, B.; He, X.; Li, K.; Li, Z.; McGee, L. R.; Pattaropong, V.; Faulder, P.; Seganish, J. L.; Shin, Y. WO Patent 2009158011. b Pan, L.; Jiang, Y.; Liu, Z.; Liu, X.-H.; Liu, Z.; Wang, G.; Li, Z.-M.; Wang, D. Eur. J. Med. Chem. 2012, 50, 18. c Sun, Z.; Wang, H.; Wen, K.; Li, Y.; Fan, E. J. Org. Chem. 2011, 76, 4149. d Patel, S.; Modi, P.; Ranjan, V.; Chhabria, M. Bioorg. Chem. 2018, 78, 258.
(a) Reichelt, A.; Bailis, J. M.; Bartberger, M. D.; Yao, G.; Shu, H.; Kaller, M. R.; Allen, J. G.; Weidner, M. F.; Keegan, K. S.; Dao, J. H. Eur. J. Med. Chem. 2014, 80, 364. (b) Walker, S. R.; Czyz, M. L.; Morris, J. C. Org. Lett. 2014, 16, 708.
Erkin, A. V.; Krutikov, V. I. Russ. J. Gen. Chem. 2012, 82, 1567. [Zh. Obsh. Khim. 2012, 82, 1532.]
Okafor, C. O. J. Org. Chem. 1973, 38, 4386.
Erkin, A. V.; Gurzhii, V. V.; Krutikov, V. I. Russ. J. Gen. Chem. 2015, 85, 79. [Zh. Obshch. Khim. 2015, 85, 86.]
(a) Aydıner, B.; Seferoğlu, Z. Eur. J. Org. Chem. 2018, 5921. (b) Santoso, K. T.; Cheung, C.-Y.; Hards, K.; Cook, G. M.; Stocker, B. L.; Timmer, M. S. M. Chem.–Asian J. 2019, 14, 1278.
Savall, B. M.; Chavez, F.; Tays, K.; Dunford, P. J.; Cowden, J. M.; Hack, M. D.; Wolin, R. L.; Thurmond, R. L.; Edwards, J. P. J. Med. Chem. 2014, 57, 2429.
Hernández Franco, L.; Palermo, J. A. Chem. Pharm. Bull. 2003, 51, 975.
Singh, U.; Chashoo, G.; Khan, S. U.; Mahajan, P.; Nargotra, A.; Mahajan, G.; Singh, A.; Sharma, A.; Mintoo, M. J.; Guru, S. K.; Aruri, H.; Thatikonda, T.; Sahu, P.; Chibber, P.; Kumar, V.; Mir, S. A.; Bharate, S. S.; Madishetti, S.; Nandi, U.; Singh, G.; Mondhe, M. D.; Bhushan, S.; Malik, F.; Mignani, S.; Vishwakarma R. A.; Singh, P. P. J. Med. Chem. 2017, 60, 9470.
Harris, C. M.; Ericksson, A. M.; Argiriadi, M. A.; Barberis, C.; Borhani, D. W.; Burchat, A.; Calderwood, D. J.; Cunha, G. A.; Dixon, R. W.; Frank, K. E.; Johnson, E. F.; Kamens, J.; Kwak, S.; Li, B.; Mullen, K. D.; Perron, D. C.; Wang, L.; Wishart, N.; Wo, X.; Zhang, X.; Zmetra, T. R.; Talanian, R. V. Bioorg. Med. Chem. Lett. 2010, 20, 334.
Bryan, M. C.; Falsey, J. R.; Frohn, M.; Reichelt, A.; Yao, G.; Bartberger, M. D.; Bailis, J. M.; Zalameda, L.; Miguel, T. S.; Doherty, E. M.; Allen, J. G. Bioorg. Med. Chem. Lett. 2013, 23, 2056.
Mazur, I. А.; Mandrichenko, B. E.; Katkevich, R. I. Usp. Khim. 1977, 46, 1233.
(a) Zhu, Y.-F.; Gross, T. D.; Gao, Y.; Connors, P. J., Jr.; Guo, Z.; Chen, C. WO Patent 2001029044; Chem. Abstr. 2001, 134, 311224. (b) Zhou, J. P.; Ding, Y. W.; Zhang, H. B.; Xu, L.; Dai, Y. Chin. Chem. Lett. 2008, 19, 669.
Matschay, A.; Skwarski, D.; Sobiak, S. Pol. J. Chem. 2000, 74, 1707.
Zhu, Y.-F.; Guo, Z.; Gross, T. D.; Gao, Y.; Connors, P. J., Jr.; Struthers, R. S.; Xie, Q.; Tucci, F. C.; Reinhart, G. J.; Wu, D.; Saunders, J.; Chen, C. J. Med. Chem. 2003, 46, 1769.
Hirai, K.; Uchida, A.; Okano, N.; Yoshizawa, T.; Yoshino, Y.; Ota, C.; Natsume, F.; Katagiri, N.; Ikeda, O.; Kawaguchi, S. JP Patent 2000264888; Chem. Abstr. 2000, 133, 252502.
Font, D.; Linden, A.; Heras, M.; Villalgordo, J. M. Tetrahedron 2006, 62, 1433.
Aakeröy, C. B.; Beffert, K.; Desper, J.; Elisabeth, E. Cryst. Growth Des. 2003, 3, 837.
Erkin, A. V.; Krutikov, V. I.; Kosykh, A. V. Russ. J. Gen. Chem. 2005, 75, 1812. [Zh. Obsh. Khim. 2005, 75, 1898.]
Nagender, P.; Reddy, G. M.; Rao, P. S.; Kurumurthy, C.; Rao, P. S.; Narsaiah, B. Chem. Lett. 2013, 42, 1018.
Laneri, S.; Sacchi, A.; Abignente, E. J. Heterocycl. Chem. 2000, 37, 1265.
Zhu, Y.-F.; Gross, T. D.; Gao, Y.; Connors, P. J., Jr.; Guo, Z.; Chen, C. WO Patent 2001029044; Chem. Abstr. 2001, 134, 311224.
Gross, T. D.; Zhu, Y.-F.; Saunders, J.; Wilcoxen, K. M.; Gao, Y.; Connors, P. J., Jr.; Guo, Z.; Struthers, R. S.; Reinhart, G. J.; Chen, C. Bioorg. Med. Chem. Lett. 2002, 12, 2185.
Hannah, D. R.; Sherer, E. C.; Davies, R. V.; Titman, R. B.; Laughton, C. A.; Stevens, M. F. G. Bioorg. Med. Chem. 2000, 8, 739.
Tukun, F.-L.; Olberg, D. E.; Riss, P. J.; Haraldsen, I.; Kaass, A.; Klaveness, J. Molecules 2017, 22, 2188.
Khanna, S.; B-Rao, S.; Ghosh, U.; Rizvi, Z.; Bajaj, K.; Kulkarni-Almeida, A.; Sharma, R. Biointerface Res. Appl. Chem. 2016, 6, 1422.
Roy, S.; Narang, B. K.; Gupta, M. K.; Abbot, V.; Singh, V.; Rawal, R. K. Drug Des. 2018, 68, 395.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(2), 103–121
Rights and permissions
About this article
Cite this article
Erkin, A.V., Krutikov, V.I. & Garabadzhiu, A.V. Priority directions in the design of biologically active compounds based on 2-aminopyrimidin-4(3H)-one and its derivatives. Chem Heterocycl Comp 57, 103–121 (2021). https://doi.org/10.1007/s10593-021-02875-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02875-w