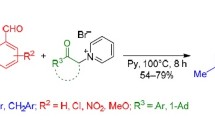
The reaction of 2-(3-cyano-5-hydroxy-1,5-dihydro-2H-pyrrol-2-ylidene)malononitriles with an excess of sodium borohydride resulted in diastereoselective formation of 2,3-diaryl-substituted 4,6-diamino-2,3-dihydrofuro[2,3-b]pyridine-5-carbonitriles. This process was accompanied by opening of the pyrrole ring in the starting compounds, followed by a double reduction and tandem closure of furan and pyridine rings.

Similar content being viewed by others
References
Fitch, R. W.; Spande, T. F.; Garraffo, H. M.; Yeh, H. J. C; Daly, J. W. J. Nat. Prod. 2010, 73, 331.
Zhou, Q.; Snider, B. B. Org. Lett. 2011, 13, 526.
Gordon, J. C.; Phillips, E.; Gurley, D. A.; Heys, J. R.; Lazor, L. A.; Barthlow, H. G.; Mallamaci, M. A.; Keith, R. A. Eur. J. Pharmacol. 2010, 645, 63.
Koike, T.; Hoashi, Y.; Takai, T.; Uchikawa, O. Tetrahedron Lett. 2011, 52, 3009.
Elmore, C. S.; Landvatter, S.; Dorff, P. N.; Powell, M. E.; Killick, D.; Blake, T.; Hall, J.; Heys, J. R.; Harding, J.; Urbanek, R.; Ernst, G. J. Labelled Compd. Radiopharm. 2014, 57, 342.
Bardasov, I. N.; Alekseeva, A. U.; Ershov, O. V.; Belikov, M. Yu. Tetrahedron Lett. 2015, 56, 5434.
Alekseeva, A. Yu.; Bardasov, I. N.; Mihailov, D. L.; Ershov, O. V. Russ. J. Org. Chem. 2017, 53, 1243. [Zh. Org. Khim. 2017, 53, 1227.]
Bardasov, I. N.; Alekseeva, A. U.; Mihailov, D. L.; Ershov, O. V.; Grishanov, D. A. Tetrahedron Lett. 2015, 56, 1830.
Ducker, J. W.; Williams, B. K. Aust. J. Chem. 1978, 31, 2327.
Belikov, M. Yu.; Fedoseev, S. V.; Ievlev, M. Yu.; Ershov, O. V. Chem. Heterocycl. Compd. 2017, 53, 1057. [Khim. Geterotsikl. Soedin. 2017, 53, 1057.]
Belikov, M. Yu.; Fedoseev, S. V.; Ershov, O. V.; Ievlev, M. Yu.; Tafeenko, V. A. Tetrahedron Lett. 2016, 57, 4101.
Zhu, D.; Ma, J.; Luo, K.; Fu, H.; Zhang, L.; Zhu, S. Angew. Chem. 2016, 128, 8592.
Zhang, J.; Zhang, J.; Kang, Y.; Shi, J.; Yao, C. Synlett 2016, 1587.
Kojima, T.; Kawajiri, I.; Nishida, J.-i.; Kitamura, C.; Kurata, H.; Tanaka, M.; Ikeda, H.; Kawase, T. Bull. Chem. Soc. Jpn. 2016, 89, 931.
Saleeb, M.; Mojica, S.; Eriksson, A.; Andersson, C. D.; Gylfe, Å.; Elofsson, M. Eur. J. Med. Chem. 2018, 143, 1077.
Engler, T. A.; Gfesser, G. A.; Draney, B. W. J. Org. Chem. 1995, 60, 3700.
The reported study was funded by the Russian Foundation for Basic Research (according to the research project No. 16-33-60156 mol_a_dk).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(4), 447–450
Rights and permissions
About this article
Cite this article
Belikov, M.Y., Fedoseev, S.V., Ievlev, M.Y. et al. New approach to the synthesis of 2,3-dihydrofuro[2,3-b]pyridine derivatives: double reduction and double heterocyclization of 2-(3-cyano-5-hydroxy-1,5-dihydro-2H-pyrrol-2-ylidene)malononitriles in the presence of sodium borohydride. Chem Heterocycl Comp 54, 447–450 (2018). https://doi.org/10.1007/s10593-018-2287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2287-x