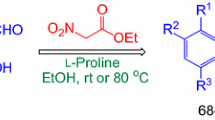
The reaction of aromatic aldehydes with Meldrum's acid and malononitrile dimer in the presence of triethylamine led to the formation of (4-aryl-3-cyano-6-oxo-1,4,5,6-tetrahydropyridin-2-yl)malononitrile triethylammonium salts, which were converted upon acidification to (4-aryl-3-cyano-6-oxopiperidin-2-ylidene)malononitriles. The reaction of these compounds with thioglycolic acid anilide was observed to produce derivatives of 1,6-naphthyridine or thieno[2,3-h][1,6]naphthyridine, depending on the conditions. Structures of (3-cyano-6-oxo-4-phenylpiperidin-2-ylidene)malononitrile and its triethylammonium salt were studied by X-ray structural analysis.





Similar content being viewed by others
References
Fuentes, L.; Bonilla, L. A.; Contreras, M. C.; Lorenzo, M. J. Synth. Commun. 1992, 22, 2053.
Daboun, H. A.; Abdou, S. E.; Khader, M. M. Heterocycles 1982, 19, 1925.
Daboun, H. A.; Abdou, S. E.; Khader, M. M. Arch. Pharm. 1983, 316, 564.
Elnadgi, M. H.; Erian, A. W. W. Arch. Pharm. 1991, 324, 853.
Abu-Shanab, F. A.; Hessen, A. M.; Mousa, S. A. S. J. Heterocycl. Chem. 2007, 44, 787.
Victory, P.; Busquets, N.; Borrell, J. I.; Teixidó, J.; Serra, B.; Matallana, J. L.; Junek, H.; Sterk, H. Heterocycles 1995, 41, 1013.
Dyachenko, I. V.; Rusanov, E. B.; Gutov, A. V.; Vovk, M. V. Russ. J. Gen. Chem. 2013, 83, 1383. [Zh. Obshch. Khim. 2013, 83, 1132.]
Dyachenko, I. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2012, 48, 1574. [Khim. Geterotsikl. Soedin. 2012, 1685.]
Junek, H. Monatsh. Chem. 1965, 96, 2046.
Koitz, G.; Thierrichter, B.; Junek, H. Heterocycles 1983, 20, 2405.
Teixidó, J.; Borrell, J. I.; Serra, B.; Matallana, J. L.; Colominas, C.; Carrión, F.; Pascual, R.; Falcó, J. L.; Batllori, X. Heterocycles 1999, 50, 739.
Victory, P. J.; Teixidó, J.; Borrell, J. I. Heterocycles 1992, 34, 1905.
Carrión, F.; Mont, N.; Batllori, X.; Borrell, J. I.; Teixidó, J. Tetrahedron 2007, 63, 215.
Carrión, F.; Pettersson, S. H.; Rifá, J.; Farran, J.; Batllori, X.; Borrell, J. I.; Teixidó, J. Mol. Diversity 2010, 14, 755.
Moustafa, M. S.; Al-Mousawi, S. M.; Hilmy, N. M.; Ibrahim, Y. A.; Liermann, J. C.; Meier, H.; Elnagdi, M. H. Molecules 2013, 18, 276.
Helmy, N. M.; El-Baih, F. E. M.; Al-Alshaikh, M. A.; Moustafa, M. S. Molecules 2011, 16, 298.
Fadda, A. A.; Refat, H. M. Monatsh. Chem. 1999, 130, 1487.
Fahmy, S. M.; Abd Allah, S. O.; Mohareb, R. M. Synthesis 1984, 976.
Abdelrazek, F. M.; Michael, F. A.; Mohamed, A. E. Arch. Pharm. 2006, 339, 305.
Fuentes, L.; Vaquero, J. J.; Soto, J. L. Synthesis 1982, 320.
Hassanien, A. Z. A.; Ghozlan, S. A. S.; Elnagdi, M. H. J. Heterocycl. Chem. 2003, 40, 225.
Elgemeie, G. E. H.; Hanfy, N.; Hopf, H.; Jones, P. G. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1998, C54, 820.
Elgemeie, G. H.; Metwally, N. H. J. Chem. Res., Synop. 1999, 208.
Abu-Shanab, F. A.; Redhouse, A. D.; Thompson, J. R.; Wakefield, B. J. Synthesis 1995, 557.
Sharanin, Yu. A.; Krivokolysko, S. G.; Dyachenko, V. D. Russ. J. Org. Chem. 1994, 30, 620. [Zh. Org. Khim. 1994, 30, 581.]
Sharanin, Yu. A.; Baskakov, Yu. A.; Abramenko, Yu. T.; Putsykin Yu. G.; Nazarova, E. B.; Vasilyev, A. F. J. Org. Chem. USSR 1984, 20, 1373. [Zh. Org. Khim. 1984, 20, 1508.]
Sharanin, Yu. A.; Khoroshilov, G. E.; Nefedov, O. M.; Litvinov, V. P.; Shestopalov, A. M. J. Org. Chem. USSR 1989, 25, 1182. [Zh. Org. Khim. 1989, 25, 1315.]
Shestopalov, A. M.; Rodinovskaya, L. A.; Litvinov, V. P.; Sharanin, Yu. A. Dokl. Chem. 1990, 314, 271. [Dokl. Akad. Nauk SSSR, Ser. Khim. 1990, 314(4), 870.]
Freeman, F. Chem. Rev. 1969, 69, 591.
Fatiadi, A. J. Synthesis 1978, 165.
Sharanin, Yu. A.; Promonenkov, V. K.; Litvinov, V. P. in Results of Science and Technology. Organic Chemistry, Shvekhgeimer, M.-G. A., Ed.; VINITI: Moscow, 1991, Vol. 20 (Part 2), p. 59.
Dyachenko, V. D.; Krivokolysko, S. G.; Litvinov, V. P. Russ. Chem. Bull., Int. Ed. 1997, 46, 1912. [Izv. Akad. Nauk, Ser. Khim. 1997, 2016.]
Dyachenko, V. D.; Krivokolysko, S. G.; Litvinov, V. P. Russ. Chem. Bull., Int. Ed. 1997, 46, 1758. [Izv. Akad. Nauk, Ser. Khim. 1997, 1852.]
Nesterov, V. N.; Krivokolysko, S. G.; Dotsenko, V. V.; Litvinov, V. P. Russ. Chem. Bull., Int. Ed. 1997, 46, 990. [Izv. Akad. Nauk, Ser. Khim. 1997, 1029.]
Krivokolysko, S. G.; Chernega, A. N.; Litvinov, V. P. Chem. Heterocycl. Compd. 2002, 38, 1269. [Khim. Geterotsikl. Soedin. 2002, 1438.]
Dotsenko, V. V.; Krivokolysko, S. G.; Chernega, A. N.; Litvinov, V. P. Dokl. Chem. 2003, 389(4–6), 92. [Dokl. Akad. Nauk 2003, 389(6), 763.]
Dotsenko, V. V.; Krivokolysko, S. G.; Chernega, A. N.; Litvinov, V. P. Russ. Chem. Bull., Int. Ed. 2003, 52, 969. [Izv. Akad. Nauk, Ser. Khim. 2003, 918.]
Frolov, K. A.; Dotsenko, V. V.; Krivokolysko, S. G.; Litvinov, V. P. Russ. Chem. Bull., Int. Ed. 2005, 54, 1335. [Izv. Akad. Nauk, Ser. Khim. 2005, 1297.]
Dotsenko, V. V.; Krivokolysko, S. G.; Litvinov, V. P. Russ. Chem. Bull., Int. Ed. 2012, 61, 131. [Izv. Akad. Nauk, Ser. Khim. 2012, 129.]
Dotsenko, V. V.; Lebedeva, I. A.; Krivokolysko, S. G.; Povstyanoi, M. V.; Povstyanoi, V. M.; Kostyrko, E. O. Chem. Heterocycl. Compd. 2012, 48, 462. [Khim. Geterotsikl. Soedin. 2012, 492.]
Frolov, K. A.; Dotsenko, V. V.; Krivokolysko, S. G. Chem. Heterocycl. Compd. 2012, 48, 1006. [Khim. Geterotsikl. Soedin. 2012, 1083.]
Osolodkin, D. I.; Kozlovskaya, L. I.; Dueva, E. V.; Dotsenko, V. V.; Rogova, Y. V.; Frolov, K. A.; Krivokolysko, S. G.; Romanova, E. G.; Morozov, A. S.; Karganova, G. G.; Palyulin, V. A.; Pentkovski, V. M.; Zefirov, N. S. ACS Med. Chem. Lett. 2013, 4(9), 869.
Dotsenko, V. V.; Frolov, K. A.; Pekhtereva, T. M.; Papaianina, O. S.; Suykov, S. Yu.; Krivokolysko, S. G. ACS Comb. Sci. 2014, 16, 543.
Ivanov, A. S.; Tugusheva, N. Z.; Solov'eva, N. P.; Granik, V. G. Russ. Chem. Bull., Int. Ed. 2002, 51, 2121. [Izv. Akad. Nauk, Ser. Khim. 2002, 1966.]
Ivanov, A. S.; Tugusheva, N. Z.; Alekseeva, L. M.; Granik, V. G. Russ. Chem. Bull., Int. Ed. 2003, 52, 1182. [Izv. Akad. Nauk, Ser. Khim. 2003, 1120.]
Pinheiro, L. C. S.; Borges, J. C.; Oliveira, C. D.; Ferreira, V. F.; Romeiro, G. A.; Marques, I. P.; Abreu, P. A.; Frugulheti, I. C. P. P.; Rodrigues, C. R.; Albuquerque, M. G.; Castro, H. C.; Bernardino, A. M. R. ARKIVOC 2008, (xiv), 77.
Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. Chemistry of Thienopyridines and Related Systems; Belen'kii, L. I., Ed.; Nauka: Moscow, 2006.
Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. Adv. Heterocycl. Chem. 2007, 93, 117.
Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. Russ. Chem. Bull., Int. Ed. 2005, 54, 864. [Izv. Akad. Nauk, Ser. Khim. 2005, 847.]
Bakhite, E. A.-G. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 929.
Brown, D. J. In The Chemistry of Heterocyclic Compounds, Taylor, E. C; Wipf, P., Eds.; John Wiley and Sons: Hoboken (USA), 2008, Vol. 63, pp. 69, 125.
Litvinov, V. P. Adv. Heterocycl. Chem. 2006, 91, 189.
Litvinov, V. P.; Roman, S. V.; Dyachenko, V. D. Russ. Chem. Rev. 2000, 69, 201. [Usp. Khim. 2000, 69, 218.]
Paudler, W. W.; Kress, T. J. Adv. Heterocycl. Chem. 1970, 11, 123.
Paudler, W.; Sheets, R. Adv. Heterocycl. Chem. 1983, 33, 147.
Mittelbach, M. Monatsh. Chem. 1985, 116, 689.
Nesterova, I. N.; Shanazarov, A. K.; Poznyak, A. M.; Lakoza, M. I.; Shemeryankin, B. V.; Granik, V. G. Pharm. Chem. J. 1994, 28, 583. [Khim.-Farm. Zh. 1994, 28(8), 41.]
Sheldrick, G. M. SHELXTL, v. 6.12, Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin, USA, 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Supplementary Iiformation file containing spectra of compounds, as well as the X-ray crystallographic data for compounds 6a, 7a, is available at http://link.springer.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(7), 473–483
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 10289 kb)
Rights and permissions
About this article
Cite this article
Dotsenko, V.V., Ismiev, A.I., Khrustaleva, A.N. et al. Synthesis, structure, and reactions of (4-aryl-3-cyano-6-oxopiperidin-2-ylidene)malononitriles. Chem Heterocycl Comp 52, 473–483 (2016). https://doi.org/10.1007/s10593-016-1918-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1918-3