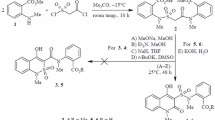
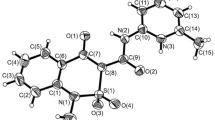
The ethyl ester of 7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-6-carboxylic acid reaction with bromine in anhydrous acetic acid producing a mixture of the 9-bromo-substituted product and 6-ethoxycarbonyl-5,7-dihydroxy-2,3-dihydro-1H-pyrido[3,2,1-ij]quinolinium tribromide in a 1:1 ratio. It has been established experimentally that the diuretic activity of 9-bromo-7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-6-carboxanilides increases substantially in comparison with their non-brominated analogs.

Similar content being viewed by others
References
I. V. Ukrainets, O. V. Gorokhova, X. V. Andreeva, and G. Sim, Int. J. Pharm. Pharmacol., 1, No. 3, 34 (2012).
I. V. Ukrainets, N. Yu. Golik, I. N. Chernenok, S. V. Shishkina, and V. A. Parshikov, Khim. Geterotsikl. Soedin., 1780 (2012). [Chem. Heterocycl. Compd., 48, 1665 (2013)].
I. V. Ukrainets, L. A. Petrushova, L. V. Sidorenko, V. B. Rybakov, and V. V. Chernyshev, Zh. Org. Farm. Khim., 2, No. 3 (7), 26 (2004).
V. B. Rybakov, S. V. Shishkina, I. V. Ukrainets, N. Yu. Golik, and I. N. Chernenok, Acta Crystallogr., Sect. E: Struct. Rep. Online, E69, o82 (2013).
D. G. Kim., Izv. Chelyabin. Nauch. Centra, Issue 1 (18), 104 (2003).
D. Długosz, M. Pach, A. Zabrzeńska, M. Zegar, B. J. Oleksyn, J. Kalinowska-Tłuścik, and K. Ostrowska, Monatsh. Chem., 139, 543 (2008).
H.-B. Burgi and J. D. Dunitz, Structure Correlation, Vol. 2, VCH, Weinheim (1994), p. 741.
Cambridge Structural Database, Cambridge Crystallographic Center: Cambridge, UK, Ver. 5.33, (2010) //www.ccdc.cam.ac.uk.
I. V. Ukrainets, N. Yu. Golik, and V. N. Kravchenko, Ukr. Pat. 98869 (2012).
P. B. Terent’ev and A. P. Stankyavichyus, Mass Spectrometric Analysis of Biologically Active Nitrogen Bases [in Russian], Mokslas, Vilnyus (1987), p. 255.
I. V. Ukrainets, N. Yu. Golik, K. V. Andreeva, and O. V. Gorokhova, Khim. Geterotsikl. Soedin., 1806 (2010). [Chem. Heterocycl. Compd., 46, 1459 (2011)] .
I. V. Ukrainets, I. N. Chernenok, N. Yu. Golik, and V. N. Kravchenko, Int. J. Pharm. Pharmacol., 1, No. 2, 19 (2012).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., A64, 112 (2008).
H. G. Vogel (editor), Drug Discovery and Evaluation: Pharmacological Assays, 3rd edition, Springer, Berlin (2008), p. 459.
Author information
Authors and Affiliations
Corresponding author
Additional information
*For Communication 232, see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1420-1427, September, 2013.
Rights and permissions
About this article
Cite this article
Ukrainets, I.V., Golik, N.Y. & Chernenok, I.N. 4-Hydroxy-2-Quinolones. 233*. Synthesis and Diuretic Activity of 9-Bromo-7-Hydroxy-5-Oxo-2,3-Dihydro-1H,5H-Pyrido[3,2,1-ij]Quinoline-6-Carboxylic Acid Anilides. Chem Heterocycl Comp 49, 1323–1330 (2013). https://doi.org/10.1007/s10593-013-1381-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1381-3