Abstract
Human activities often induce maladaptive effects in urban wildlife, such as increased inbreeding and decreased gene flow, consequently leading to a loss of biodiversity. Although maladaptive effects are commonly reported in urban wildlife, some wildlife species are not affected. Understanding the conditions and factors that affect the genetic structure of wildlife is crucial for its conservation in urban environments. Eurasian red squirrels Sciurus vulgaris in Obihiro City, Hokkaido, Japan, have settled in large residential lots with a high risk of vehicle collisions for approximately 30 years, raising the possibility that the urban population would be genetically isolated from nearby rural populations and may experience a rapid decrease in genetic diversity. In this study, we analysed the population structure, genetic distance, and nucleotide diversity of squirrels in the Obihiro area using genome-wide single nucleotide polymorphisms (SNPs). Principal component analysis and ADMIXTURE showed that urban and rural squirrel populations formed distinct clusters with intermediate urban and rural boundary populations. Genetic distances between the urban populations were greater than that of other pairs. Nucleotide diversities were lower in urban populations than in other populations, suggesting limited gene flow between urban and other populations, possibly due to urbanisation. The change in the genetic characteristics of urban squirrel populations during this short period demonstrates the strong impact of urbanisation. Our findings suggest that a genome-wide approach to the genetic structure of wildlife contributes to its conservation in urban environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, urban areas have dramatically expanded around the world, providing alternative habitats for wildlife to natural ones (Lowry et al. 2013; Johnson et al. 2020). Some wildlife species have successfully flourished in urban areas by altering their ecological traits (Santini et al. 2019; Shutt and Lees 2021). However, even species living in urban areas experience increased inbreeding and decreased genetic diversity (Schmidt and Garroway 2021). These negative impacts mainly result from impervious surfaces (e.g., urban infrastructure, roads, and buildings) in urban environments, which hamper dispersal and induce habitat fragmentation (Hulme-Beaman et al. 2016; Munshi-South et al. 2016), consequently inducing genetic differentiation between populations. Genetic differentiation caused by urban factors has been termed as the “urban fragmentation model”, and has been tested and supported in urban evolutionary studies (Crispo et al. 2011; Miles et al. 2019). Although the urban fragmentation model was the most commonly reported pattern of urban evolutionary consequence, an inverse pattern referred to as the “urban facilitation model” has also been reported (Björklund et al. 2009; Miles et al. 2018). For example, the urban populations of the Western black widow spider Latrodectus hesperus and the great tit Parus major have higher genetic diversity thanSchmidt & Garroway 2021 forest populations (Björklund et al. 2009; Miles et al. 2018). Thus, the factors and conditions that affect genetic structure in urban environments would vary by region and species.
According to the review by Fusco et al. (2021) several factors must be considered when developing the field of population genetic structure in urban environments, such as molecular methods and environmental variations. Previous studies have used microsatellite markers to identify genetic differentiation (Rézouki et al. 2014; Selonen et al. 2018). However, this has low detectability of genetic differentiation because of the use of a small number of loci (McCartney-Melstad et al. 2018; Fusco et al. 2021). In addition, because geographic patterns and landscapes would differ between countries, there are differences in the effects on population genetic structure (Johnson and Munshi-South 2017; Fusco et al. 2021). Most studies have been conducted in North America and Europe, with fewer studies from other regions (e.g., Asia, Africa, and South America) with differing urban developments, socioeconomics, and human cultures. In addition, various approaches such as behaviors and life history as well as genetic structure may be useful to better understand the mechanism and factors shaping the population genetic structure in urban areas. However, the urban ecology of only a few species has been understood from multiple perspectives.
Eurasian red squirrels Sciurus vulgaris are the appropriate animals to identify the urban factors that affect their population structures because their urban ecology has been well studied in the last decade pertaining broad perspectives such as diet (Krauze-Gryz et al. 2021; Takahata et al. 2023), cause of mortality (Blackett et al. 2018), habituation to humans (Uchida et al. 2016, 2019), dispersal (Fey et al. 2016; Hämäläinen et al. 2019), as well as population genetic structure (Rézouki et al. 2016; Selonen et al. 2018). Considering the natural history of urban squirrels described below, they might be vulnerable to urbanisation, and genetic differentiation between urban populations could easily occur. Firstly, the presence of roads would hamper squirrel dispersal because collisions with vehicles are causing the highest number of mortalities in urban squirrels (Blackett et al. 2018; Fingland et al. 2022). Secondly, landscapes and anthropogenic resources in urban environments would affect squirrel dispersal. For example, the dispersal distances and routes of squirrels are affected by the presence of open spaces (without tree canopy cover, i.e., a high risk of predation), such as agricultural fields in urban areas (Hämäläinen et al. 2019). In addition, they are usually fed by citizens in the parks and private gardens (Krauze-Gryz et al. 2021; Uchida et al. 2021; Takahata et al. 2023), and this supplemental feeding might affect their decision to disperse and the dispersal distances (Selonen et al. 2018). Therefore, urban environments contain numerous factors that hamper squirrel dispersal, induce habitat fragmentation, and increase genetic differentiation between populations.
Although habitat fragmentation and genetic differentiation had been assumed to influence urban squirrels, previous studies investigating their population structure have shown little evidence of those consequences. For example, in the urban population in Finland, although genetic differentiation increased within urban populations, there was no indication that urban populations were isolated from rural populations (Selonen et al. 2018). Also in France, urban populations were not affected by urbanisation because the urban population had high genetic diversity and low inbreeding coefficients (Rézouki et al. 2016). However, these studies were conducted at urban sites where conditions that facilitated squirrel movements and dispersals were maintained. The Finnish study was conducted in a small city (Turku, approximately 3 × 4 km2 area) surrounded by forests and numerous urban green spaces. Similarly, the French study was conducted in an urban green space surrounded by residential areas and adjacent to a large forest area. Although these study sites are located in urban areas, squirrels were studied at sites where they could easily disperse to other habitats and maintain gene flow. Therefore, the population genetic structure of urban squirrels could differ in environments with different degrees of urbanisation and occupancy of green spaces. Alternatively, it is possible that the previous studies using microsatellite markers could not detect the genetic differentiation (Rézouki et al. 2016; Selonen et al. 2018).
The Obihiro City in Hokkaido, Japan is one of the areas where squirrels live in urban environments (Uchida et al. 2019; Shimamoto et al. 2020; Takahata et al. 2023). In Obihiro City, large residential lots (approximately 13 × 7 km2 area) were constructed, and squirrel habitats such as urban parks, groves, and roadside trees are scattered throughout the urban areas. These habitats are at least 1 km apart from the others, and there are few forests that connect each squirrel habitat (Fig. 1). In addition, the presence of traffic roads may hamper squirrel’s dispersal because they are frequently killed by vehicle collisions (Yanagawa 2005). Therefore, urban squirrels in Obihiro City can be expected to be genetically different from those in adjacent populations in rural and natural areas. Genetic differentiation also occurs in urban populations. That is, squirrels in Obihiro City would have different outcomes in terms of the genetic structure compared to previous study findings (Rézouki et al. 2016; Selonen et al. 2018). However, the genetic structure of squirrels in the Obihiro City remains unknown.
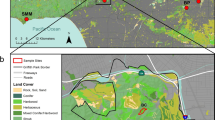
Map of Hokkaido and the Obihiro City with 12 study sites. Urban-central, urban-edge, and rural study sites are plotted as each shape and colour (urban-central: blue circle, urban-edge: yellow squire, rural: green triangle). Residential lots are shown as white, agriculture lands are shown as yellow, brown, and shallow green. Forests including windbreak forests are shown as deep green
In this study, we used genome-wide single nucleotide polymorphisms (SNPs) to investigate the population structure and genetic diversity of Eurasian red squirrels in and around the Obihiro City (hereafter, Obihiro area in this study) of Hokkaido, Japan. We obtained SNPs data by MIG-seq (Suyama and Matsuki 2015) and focused on three aspects; (1) population structure: we used principal component analysis (PCA) and ADMIXTURE to investigate the population structure; (2) genetic differentiation: we used pairwise FST values between populations (study sites) to find environmental factors affecting genetic differentiation; and (3) genetic diversity: we used nucleotide diversity (π) evaluated from spatial-temporal standpoint as well as sex differences to investigate factors affecting their genetic diversity. In this study, we predicted that genetic differentiation would occur between urban and rural populations, as well as within urban populations, owing to decreased gene flow in urban populations. From a spatial standpoint of genetic diversity, we predicted that urban populations would have a lower nucleotide diversity than rural populations. From a temporal standpoint, we predicted that nucleotide diversity would decrease faster in urban populations than in rural populations. Regarding the effect of sex, we predicted that males and females have similar nucleotide diversity because they do not have a sexual bias in dispersal (Wauters et al. 2010mäläinen et al. 2019).
Materials and methods
Study sites
Data sampling was conducted in the Obihiro area of Hokkaido, Japan (42°49′ N, 143°02′ E). Obihiro City is composed of large residential lots surrounded by agricultural land that was cultivated 140 years ago. Within the city, urban green spaces (such as urban parks, windbreak forests, and groves) are scattered and serve as habitats for squirrels (Fig. 1). Squirrels have settled in urban green spaces as forests have matured (Ezorisu no kai 1989), and a stable population has been observed over the past 30 years.
We selected 12 squirrel habitats located in and around Obihiro City as study sites. We defined squirrels that inhabit those study sites as “urban-central”, “urban-edge”, and “rural” populations in order to calculate land use percentages (Table 1; Fig. 1) using QGIS (QGIS Development Core Team 2023) in this study. Land use data (100 × 100 m mesh map with 12 land use categories, such as residential lots, roads, agricultural land, forests, and rivers) were downloaded from the Ministry of Land, Infrastructure, Transport, and Tourism (MLIT 2016). In QGIS, we created a point at the centre of each study site and a 1 km radius area of the centre point. We then overlapped the land use data to 1 km radius areas and calculated the number of meshes belonging to each land use category in the number of meshes within the radius areas. We excluded the number of meshes belonging to the study sites because we calculated land use percentages to define the environment surrounding the study sites.
Six forests located at least 10 km far from central Obihiro city and surrounded by agricultural lands with few roads were selected as “rural” sites (mean and SE for area of study sites was 2.75 ± 0.65 ha; Table 1; Fig. 1). Although rural sites included human-mediated sites, such as shrines or horse-riding parks, all sites were rarely visited by humans. Because agricultural lands were mainly occupied in rural sites (Table 1), many windbreak forests comprising Korean pine, Pinus koraiensis or Japanese larch, Larix kaempferi distributed such that they served as corridors between sites.
Two urban parks located within residential areas were selected as “urban-central” sites (here after; “central”, mean and SE for area of study sites was 25.83 ± 17.42 ha; Table 1; Fig. 1). The central sites were mainly surrounded by residential lots and roads (more than 90%), and there were few groves and forests (Table 1; Fig. 1). Thus, many squirrels were killed by vehicle collisions during dispersal or movement (Yanagawa 2005). Central sites were frequently visited by humans (mean and SE for the number of human visitors in 30 min: 9.83 ± 0.09, see below). In addition, supplemental feeding of wildlife is a common activity in central sites (Uchida et al. 2021; Takahata et al. 2023). Food availability for squirrels (particularly tree seeds) is more stable in central than in rural study sites because of the presence of supplemental feeding (Takahata et al. 2023).
Three urban parks and the Obihiro University campus, located in residential areas, were selected as “urban-edge” sites in this study (here after; “edge”, mean and SE for area of study sites was 14.62 ± 10.23 ha; Table 1; Fig. 1). Edge sites were similar to central sites in terms of location, high land use proportion of roads and residential lots (Table 1), frequent visitation of humans (mean and SE for the number of human visitors in 30 min: 4.02 ± 0.05), and presence of supplemental food. However, there are riparian forests and windbreak forests that squirrels could use for dispersal near the edge sites. The proportion of agricultural land and forest was higher than that in central sites (Table 1). Although the edge sites were located in residential areas, they exhibit the characteristics of both central and rural sites. Therefore, in terms of the landscape and geographic characteristics which would affect the squirrel’s population genetic structure, we defined the edge site in this study (note that previous studies used urban vs. rural comparison, Uchida et al. 2016; Shimamoto et al. 2020; Takahata et al. 2023).
Data collection
Trapping of squirrels was conducted between 2015 and 2021. The protocol for capturing squirrels was the same as that used in our previous study (Takahata et al. 2023). Squirrels were captured using box traps (Model RB-2, Sakae Industry Co., Ltd., Niigata, Japan) from 4:00 am to 11:00 am in May–June and October–November when squirrels were most active. Box traps were set on the ground and checked every 1–1.5 h in rural sites, whereas they were always monitored in central and edge sites to avoid human conflict. When the squirrels were captured, we recorded their sex and attached individual identifiers (ear tags and collar rings). Also, we recorded their maturity (juvenile, subadult, or adult) by checking their body weight and genital maturity. For the DNA experiment, 2 mm round tissue was taken from their ear by using the ear punch (Model KN-292, Natsume Seisakusho Co., Ltd., Tokyo, Japan) and stored in 99% ethanol in a deep freezer (− 80 ℃). All protocols were completed within 15 min to minimise stress, and the squirrels were released at the capture location. Squirrels that had previously undergone tissue sampling were immediately released. The capture and handling of squirrels was approved by the Institutional Animal Care and Use Committee of the Graduate University for Advanced Studies, SOKENDAI (licence number: SKD2020AR002), and the Institutional Animal Care and Use Committee of the National University Corporation Hokkaido University (licence number:15–0121).
DNA extraction and MIG-seq
Genomic DNA was extracted from tissue samples (half of 2 mm round tissue) using the DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.
MIG-seq (Suyama and Matsuki 2015) was used to obtain sequence data. This method amplifies regions flanked by microsatellite loci via a two-step PCR. In the first step of PCR, Inter-Simple Sequence Repeat (ISSR) regions were amplified using eight pairs of ISSR primers (minor modification of primer sequences from Suyama and Matsuki (2015); Supplementary Material Table S1). The first PCR was conducted following Suyama and Matsuki (2015): initial activation at 94 °C for 1 min, 25 cycles for denaturation at 94 °C for 30 s, annealing at 48 °C for 1 min and extension at 72 °C for 1 min, followed by a final incubation at 72 °C for 5 min. In the second PCR, we used the NEBNext Multiplex Oligos for Illumina (New England Biolabs Inc., Massachusetts, USA) for primer pairs: initial activation at 94 °C for 1 min, 25 cycles for denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 75 s, followed by a final incubation at 72 °C for 3 min. Two-step PCR was conducted using the TaKaRa PCR Thermal Cycler Dice Touch (Model TP350; Takara Bio Inc., Shiga, Japan). After the second PCR, equal amounts of PCR products from each individual were mixed and purified using AMPure XP (Beckman Coulter). Paired-end (2 × 150 bp) sequencing (59.9 M bp–259.5 M bp) was performed on the Illumina HiSeq X platform.
SNPs calling
Sequence reads from the genomic DNA libraries of 286 squirrels (rural populations, n = 52; edge populations, n = 90; central populations, n = 144) were trimmed to remove adaptor sequences and mapped to the reference genome (mSciVul1.2) using the CLC Genomics Workbench ver. 20.0.4 (https://www.qiagenbioinformatics.com/). Reads showing high similarity (> 80% in > 80% of reads length) were mapped to the reference genome sequence and reads mapped to more than one position in the genome were removed (“ignore” option for reads mapped to multiple positions). The mapping data were exported in bam file format, sorted, and indexed using SAMtools (Li et al. 2009). The duplicated reads in the bam files were marked using the MarkDuplicates algorithm implemented in GATK v4.2 (https://gatk.broadinstitute.org/hc/en-us). Genotype calling was performed for all individuals analysed in this study using the HaplotypeCaller algorithm in GATK v4.2. The genotypes of all individuals were output in the gvcf format (-ERC GVCF option). All gvcf files were combined into a single gvcf format file using the CombineGVCF algorithm in GATK v4.2. The combined file was genotyped by the GenotypeGVCFs algorithm and filtered by Filtervcf in GATK v4.2 with parameters; --filter-expression “QD < 2.0” --filter-name “QD2” --filter-expression “QUAL < 30.0” --filter-name “QUAL30” --filter-expression “FS > 200.0” --filter-name “FS200” --filter-expression “SOR > 10.0” -filter-name “SOR10” --filter-expression “ReadPosRankSum < -20.0” --filter-name “ReadPosRankSum-20”. The genotyped vcf file was filtered using vcftools with the following options: mac 3 --maxDP 1e + 03 --minDP 3 --minGQ 20 --max-missing 0.8 (Danecek et al. 2011).
Genome-wide SNP analyses
We performed a principal component analysis (PCA) using PLINK ver. 1.9 (Purcell et al. 2007) with an option “--indep-pairwise 50 10 0.1” to examine the genetic relationship between central, edge and rural squirrels (Fig. 2). In addition, the ADMIXTURE ver. 1.3 (Alexander and Lange 2011) was run on the same PCA dataset, assuming two to five ancestral genetic compositions (K = 2–5). We calculated cross validation errors (CV error) to find the appropriate number of ancestral genetic compositions.
Population genetic structure of Eurasian red squirrels in Obihiro area. (a) Principal components analysis (PC1 vs. PC2) of 199 samples based on 655 SNPs. Urban-central samples are shown as circles, urban-edge samples are shown as squares, and rural samples are shown as triangles. (b) ADMIXTURE results based on SNPs data for K = 2. Each bar represents one individual and colors represent percentages belonging to either one of two ancestral genetic compositions (i.e., urban genetic compositions were shown as grey, and rural genetic compositions were shown as black). Areas (urban-central, urban-edge, and rural) and sites (e.g., MID, ODO, AZU) to which each individual belongs are indicated by the top and bottom of the bar, respectively
We calculated pairwise FST values using vcftools v0.1.16 (Danecek et al. 2011) to investigate genetic distances between population pairs (in total, 66 pairs, Supplementary Materials Table S2). To investigate the correlation between FST values and geographical distance, we used Mantel’s test. Geographic distances (km) between populations were measured as the shortest distance from one edge of the study site to another, using QGIS tools. We constructed a general linear model (GLM) to investigate the sites where genetic differentiation likely occurred. The pairwise FST value between populations was the dependent variable, and the geographic distances between populations and site category of pairs (urban-urban, urban-rural, rural-rural; n = 15, 36, and 15, respectively) were explanatory variables. Additionally, genetic distances standardised by geographic distance (pairwise FST/geographic distance) were calculated for each site category. Although we wanted to investigate the effects of residential lots on genetic distance, we had only two central populations (MID and ODO populations). Thus, central and edge populations were included as the “urban population” in the model because edge sites were located in residential areas similar to central sites for the calculation of pairwise FST values.
Nucleotide diversity was calculated for each population, as well as for combinations of populations between years and sex, using vcftools v0.1.16. To ensure data accuracy, populations or combinations with fewer than five samples were excluded. Because the sample size of all combinations of rural study populations and years was less than five, we combined them into a single rural area. To identify factors that affect nucleotide diversity (sites category, years, and sex), we conducted two separate GLMs for the sex (the sex model) and the year (the year model) using the “car” package in R (R Development Core Team 2023). For the sex model, nucleotide diversity was the dependent variable, and sex (female or male) and site category (central, edge, or rural) were the grouping variables. For the year model, nucleotide diversity was the dependent variable, year (2015–2021) was the covariate, and the site category (central, edge, or rural) was the grouping variable. If we found significance in the site category, we performed a post-hoc test using Tukey’s multiple comparison test. The nucleotide sequences were deposited in the DDBJ Sequenced Read Archive (accession number: PRJDB16606). All data and codes used in this study are available at https://osf.io/s6k2p/.
Results
Population genetic structure of squirrels in the Obihiro area
Initially, we investigated the population genetic structure of squirrels in the Obihiro area using PCA. The PCA results showed that central and rural squirrels formed different clusters with a slight overlap (Fig. 2a). In addition, edge squirrels were located between the clusters of central and rural squirrels along the PC1 axis (Fig. 2a). These results suggest that central and rural squirrel populations were genetically differentiated, with a limited extent of mixing through edge populations. ADMIXTURE analysis divided all individuals into two ancestral genetic compositions: one predominant in the central populations and the other predominant in the rural populations, with the lowest CV error of K = 2 (Fig. 2b). Although central and rural populations consisted of two ancestral genetic compositions with different ratios, they were genetically differentiated. The edge populations were a mixture of the two compositions (Fig. 2b), indicating a mix of central and rural populations (i.e., gene flow) through the edge populations.
Genetic distance between populations
Next, we analysed the genetic distance between populations in the Obihiro area. We used pairwise FST values between populations as genetic distances. We found that genetic distance values were significantly correlated with geographic distance (Mantel r = 0.58, P < 0.001; Fig. 3a), indicating that squirrels in the Obihiro area showed a pattern of isolation by distance. The GLM showed that pairwise FST values were affected by the site category of pairs (Fig. 3b; Table 2), indicating that the urban-urban and urban-rural categories had larger genetic distances than the rural-rural category (Fig. 3b; Table 2). The standardised genetic distances of urban-urban pairs were significantly higher than those of rural-rural pairs (Fig. 4a; Table 2). For a closer comparison of the genetic distances, we classified the 12 sites into three categories: central, edge, and rural. Although we did not perform a statistical analysis because of the small sample size in the central-central category (only one pair), pairwise central populations (central-central and central-edge sites) had higher standardised genetic distances than the other pairs (Fig. 4b). In addition, pairwise edge populations (edge-edge sites) had higher standardised genetic distances than pairwise rural populations (edge-rural, rural–rural sites). These results suggest limited gene flow between central populations and other populations and between edge populations.
Relationship among population pairwise geographic distances and population pairwise FST values. (a) Relation between geographic distances and FST values between all site category pairs (66 pairs). (b) Relation between geographic distances and FST values between each site category pairs (urban-urban: circles with solid line, urban-rural: squares with dotted line, rural-rural: triangles with dashed lines). Urban category including the urban central- and urban-edge population in this Fig. 95% credible intervals are shown in grey in each plot
Standardised genetic distances (FST/geographic distance) among pairwise site categories. (a) Box plots of standardized genetic distances between each site category pair (urban-urban, urban-rural, and rural-rural). Urban category including the urban-central and urban-edge population in this figure. (b) Box plots of standardized genetic distances between each site category pair including urban-edge areas. Horizontal lines represent medians and interquartile ranges (IQR). The upper and lower whiskers extend from the hinge to the largest and smallest value no further than 1.5 × IQR from the hinge
Nucleotide diversity
The sex model showed that nucleotide diversity was affected by the site category (GLM, F2,12 = 6.19, P = 0.01, multiple R2 = 0.51). From Tukey’s multiple comparisons of means, we found that nucleotide diversity was higher in rural squirrels than in central (P = 0.04) and edge squirrels (P = 0.01). Diversity did not differ between the central and edge areas (P = 0.95). In addition, we did not find any significant differences in sex (F1,12 = 0.26, P = 0.61; Fig. 5a).
Nucleotide diversity in each area, sex, and year. a) Box plots of nucleotide diversity in each area and sex. Females are shown as white, and males are shown as grey boxplots. Circles represent means, and horizontal lines represent medians and interquartile ranges (IQR). The upper and lower whisker extends from the hinge to the largest and smallest value no further than 1.5 × IQR from the hinge. B) Nucleotide diversity in each site category and year (2015–2021). Rural areas are shown as white shapes with the dashed line, urban-edge areas are shown as grey shapes with dotted lines, and urban-central areas are shown as black shapes with solid lines. Each shape with the same colour indicates the same study site. Some of the plots (e.g., AZU and CHI site in urban-edge areas, other sites excluding MID site in 2015) were not plotted because there were not enough sample size to calculate nucleotide diversity
The year model showed that nucleotide diversity was affected by the site category (GLM, F2,16 = 7.66, P = 0.01, multiple R2 = 0.53). Using Tukey’s multiple comparisons of means, we found that nucleotide diversity was higher in rural areas than in central areas (P = 0.01). There were no significant differences between rural and edge areas (P = 0.13) or between central and edge areas (P = 0.50). In addition, nucleotide diversity was affected by year (F1,16 = 4.68, P = 0.05; Fig. 5b), indicating that diversity decreased over the years.
Discussion
We used a genome-wide approach to investigate the genetic structure and nucleotide diversity of Eurasian red squirrels distributed in rural to urban areas in the Obihiro area of Hokkaido, Japan. We found that squirrels in the Obihiro area were genetically differentiated between urban-central and rural populations (Fig. 2a, b) with gene flow via urban-edge populations. Genetic distances between central populations were significantly greater than those between rural populations affected by isolation (Fig. 4a). This result indicates that urban factors limit the dispersal of squirrels, and thus limit the gene flow between central populations and edge and rural populations. In addition, genetic distance was remarkably higher between central populations than between edge populations (Fig. 4b), indicating that certain factors hamper squirrel dispersal and cause genetic differentiation in central areas.
We also found that the nucleotide diversity in urban populations was lower than that in rural populations (Fig. 5a, b). This is possibly due to the decreased population size caused by limited inhabited areas. Another possible explanation for the lower genetic diversity in urban populations could be the different degrees of gene flow between urban and rural populations. Because the Obihiro area is surrounded by forests (Fig. 1), rural subpopulations around the Obihiro area are connected via gene-flow, and they would have maintained genetic diversity. However, the genetic diversity of urban populations may have decreased because of restricted gene flow between the urban population and the populations around the Obihiro area. Our results showed low genetic diversity and reduced gene flow in urban populations, indicating that urbanisation would negatively affect the long-term persistence of a population. Which urbanisation factors reduce gene flow in the Obihiro area? We consider the following three factors.
One possible reason for the reduction of gene flow is the presence of busy roads. In Obihiro City, many squirrels were killed in vehicle collisions (Yanagawa 2005), and our results showed that one of the populations (the AZU population) that was separated by the arterial road had a greater genetic distance to its neighbouring populations after controlling for the effect of geographic distance (Supplementary Materials Table S2). Therefore, it is possible that traffic roads act as barriers to squirrel dispersal, consequently increasing genetic differentiation between populations. This possibility is also supported by the evidence that the main cause of fatalities in urban squirrels is vehicle collisions (Blackett et al. 2018; Fingland et al. 2022). To determine the impacts of the presence of roads on squirrel’s genetic structure, it would be fruitful to investigate whether and how the decrease in gene flow is affected by both vehicle collisions and squirrel’s dispersal in urban areas.
Secondly, population connectivity, such as the presence of forest corridors between populations, is a factor that affects gene flow. Forests, which connected populations were abundant between the rural and edge populations (Table 1). In the Obihiro area, numerous Korean pines, Japanese larches, and Sakhalin spruce Picea glehnii have been planted as windbreak forests for agricultural purposes (Tsuji et al. 2004, 2007). These windbreak forests could aid squirrel dispersal from rural to urban areas by connecting habitats and providing food (tree seeds) for squirrels. Therefore, gene flow between populations in rural and edge areas, and between populations in edge areas, may be due to the dispersal of squirrels through connective forests. In contrast, the central areas were mainly constructed with residential lots that hampered squirrel dispersal, and there were few forests or groves (Table 1). The dispersal of squirrels may be restricted because of the lack of forests in central areas. Although forests, including windbreak forests in the Obihiro area, might be a crucial factor for squirrel gene flow, windbreak forests have been cut in recent years for more efficient farming practices (Tsuji et al. 2004, 2007). Further logging would result in even less gene flow between rural and central populations, causing central populations to become more isolated. To increase the genetic potential of the meta-population that can respond to future challenges, it would be crucial to maintain green patches and forest corridors in urbanised areas to foster opportunities for squirrel dispersal.
Thirdly, the abundance of supplemental food in urban areas may alter squirrels’ decisions to disperse and the dispersal distance. Squirrels disperse when they encounter a high frequency of intraspecific competition for food and breeding resources (Wauters and Dhondt 1993). Therefore, abundant food from supplemental feeding possibly mitigates food scarcity and intraspecific competition and hampers their dispersal behaviour. Additionally, if supplemental food is widely distributed in urban green spaces, squirrels do not need to disperse over long distances (Selonen et al. 2018). Urban squirrels in Finland disperse over shorter distances than rural squirrels (Selonen et al. 2018). As we surveyed only two central and three edge sites, we were unable to detect factors that affect genetic differentiation in urban populations. In future studies, it would be beneficial to collect samples from many urban habitats with variations in the presence of roads and forests, site connectivity, food availability, and distribution.
Overall, our results suggest that genetic differentiation between rural and urban squirrel populations occurred rapidly in the Obihiro area, within 30 years of squirrels settling in urban environments. Considering the generation time of squirrels, this differentiation may have occurred within only 15–30 generations. Therefore, our results indicate that the genetic structure was strongly affected by urbanisation (including human activities and man-made landscapes) over a relatively short time.
We also investigated the effects of sex on the genetic diversity of urban squirrels. In Eurasian red squirrels, sexual bias in dispersal has not been reported (Wauters et al. 2010mäläinen et al. 2019). However, they exhibit a sexual bias in their dispersal when they encounter frequent intrasexual competition for food resources and breeding (Wauters and Dhondt 1993). In our study population, male-male competition was more frequently observed than female-female competition in both rural and urban areas (Takahata et al. unpublished data). Therefore, we hypothesized that nucleotide diversity has sex variation in both urban and rural populations. However, we did not find any significant sex-related differences in nucleotide diversity (Fig. 5a). A possible reason for this is that squirrels in our system did not have sexual bias in dispersal behaviour that is the same as in previous studies (Wauters et al. 2010mäläinen et al. 2019). Alternatively, this may be due to the lack of sexual bias in the dispersal of squirrels in the Obihiro area, which could be caused by different scenarios between rural and urban populations. In rural populations with low squirrel density, the frequency of intraspecific competition would be low, which did not induce sex differences in their dispersal behaviour. In urban populations, urban factors such as the presence of roads and a lack of forests may strongly hamper squirrel dispersal, which may have resulted in similar nucleotide diversity between the sexes even though they had sexual bias in dispersal behaviour. To verify if our results regarding the lack of sex-biased dispersal in squirrels can be generalised, additional studies on the relationship between sexual biases in genetic diversity and behaviours, such as competition and dispersal will be needed.
Our study findings suggest that urban squirrels are affected by urbanisation over the short period of 30 years. In addition, based on the findings of previous studies on the urban ecology of Eurasian red squirrels, we gained insights into the factors that possibly affect their population genetic structure. In recent years, urban green spaces have been considered places to conserve wildlife and biodiversity. For instance, European countries conduct urban planning, such as arranging green infrastructure, including wildlife corridors and green roofs, that connect wildlife habitats and maintain gene flow among populations (Lundholm 2015; Schell et al. 2021). However, when we conduct urban planning as described above and evaluate the results from those efforts, knowledge about wildlife’s urban ecology, such as dispersal behaviours, causes of mortality, and resource use, are necessary. To substantially conserve wildlife in urban environments, it is necessary to evaluate the effects on genetic structures with a deep understanding of the ecology of urban wildlife, and to consider urban planning that could contribute to their urban lives from a comprehensive perspective.
Data availability
The datasets and code used in this study are available at https://osf.io/s6k2p/. The nucleotide sequences were deposited in the DDBJ Sequenced Read Archive (accession number: PRJDB16606).
References
Alexander DH, Lange K (2011) Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform 12:246. https://doi.org/10.1186/1471-2105-12-246
Björklund M, Ruiz I, Senar JC (2009) Genetic differentiation in the urban habitat: the great tits (Parus major) of the parks of Barcelona city: genetic differentiation in Barcelona. Biol J Linn Soc 99:9–19. https://doi.org/10.1111/j.1095-8312.2009.01335.x
Blackett TA, Simpson VR, Haugland S et al (2018) Mortalities, amyloidosis and other diseases in free-living red squirrels (Sciurus vulgaris) on Jersey, Channel Islands. Vet Rec 183:503–503. https://doi.org/10.1136/vr.104779
Crispo E, Moore J, Lee-Yaw JA, Gray SM, Haller BC (2011) Broken barriers: human‐induced changes to gene flow and introgression in animals. BioEssays 33:508–518. https://doi.org/10.1002/bies.201000154
Danecek P, Auton A, Abecasis G et al (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158. https://doi.org/10.1093/bioinformatics/btr330
R Development Core Team (2023) R: A language and environment for statistical computing. Vienna, Austria. R Foundation for Statistical Computing. http://www.R-project.org/
Ezorisu no kai (1989) Report on the status of Eurasian red squirrels in the Midorigaoka park, Obihiro City. (in Japanese)
Fey K, Hämäläinen S, Selonen V (2016) Roads are no barrier for dispersing red squirrels in an urban environment. Behav Ecol 27:741–747. https://doi.org/10.1093/beheco/arv215
Fingland K, Ward SJ, Bates AJ, Bremner-Harrison S (2022) A systematic review into the suitability of urban refugia for the eurasian red squirrel Sciurus vulgaris. Mamm Rev 52:26–38. https://doi.org/10.1111/mam.12264
Fusco NA, Carlen EJ, Munshi-South J (2021) Urban Landscape Genetics: are biologists keeping up with the Pace of Urbanization? Curr Landsc Ecol Rep 6:35–45. https://doi.org/10.1007/s40823-021-00062-3
Hämäläinen S, Fey K, Selonen V (2019) The effect of landscape structure on dispersal distances of the eurasian red squirrel. Ecol Evol 9:1173–1181. https://doi.org/10.1002/ece3.4806
Hulme-Beaman A, Dobney K, Cucchi T, Searle JB (2016) An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol Evol 31:633–645. https://doi.org/10.1016/j.tree.2016.05.001
Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327. https://doi.org/10.1126/science.aam8327
Johnson HE, Lewis DL, Breck SW (2020) Individual and population fitness consequences associated with large Carnivore use of residential development. Ecosphere 11:e03098. https://doi.org/10.1002/ecs2.3098
Krauze-Gryz D, Gryz J, Brach M (2021) Spatial organization, behaviour and feeding habits of red squirrels: differences between an urban park and an urban forest. J Zool 315:69–78. https://doi.org/10.1111/jzo.12905
Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments: behavioural responses to urban environments. Biol Rev 88:537–549. https://doi.org/10.1111/brv.12012
Lundholm JT (2015) The ecology and evolution of constructed ecosystems as green infrastructure. Front Ecol Evol 3:106. https://doi.org/10.3389/fevo.2015.00106
McCartney-Melstad E, Vu JK, Shaffer HB (2018) Genomic data recover previously undetectable fragmentation effects in an endangered amphibian. Mol Ecol 27:4430–4443. https://doi.org/10.1111/mec.14892
Miles LS, Johnson JC, Dyer RJ, Verrelli BC (2018) Urbanization as a facilitator of gene flow in a human health pest. Mol Ecol 27:3219–3230. https://doi.org/10.1111/mec.14783
Miles LS, Rivkin LR, Johnson MTJ et al (2019) Gene flow and genetic drift in urban environments. Mol Ecol 28:4138–4151. https://doi.org/10.1111/mec.15221
Ministry of Land, Infrastructure, Transport, and Tourism, L03-b (2016).
Munshi-South J, Zolnik CP, Harris SE (2016) Population genomics of the Anthropocene: urbanization is negatively associated with genome‐wide variation in white‐footed mouse populations. Evol Appl 9:546–564. https://doi.org/10.1111/eva.12357
Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. https://doi.org/10.1086/519795
QGIS Development Core Team (2023) QGIS Geographic Information System. QGIS Association. http://www.qgis.org/
Rézouki C, Dozières A, Le Cœur C et al (2014) A viable population of the European red squirrel in an urban park. PLoS ONE 9:e105111. https://doi.org/10.1371/journal.pone.0105111
Santini L, González-Suárez M, Russo D et al (2019) One strategy does not fit all: determinants of urban adaptation in mammals. Ecol Lett 22:365–376. https://doi.org/10.1111/ele.13199
Schell CJ, Stanton LA, Young JK et al (2021) The evolutionary consequences of human–wildlife conflict in cities. Evol Appl 14:178–197. https://doi.org/10.1111/eva.13131
Schmidt C, Garroway CJ (2021) The population genetics of urban and rural amphibians in North America. Mol Ecol 30:3918–3929. https://doi.org/10.1111/mec.16005
Selonen V, Fey K, Hämäläinen S (2018) Increased differentiation between individuals, but no genetic isolation from adjacent rural individuals in an urban red squirrel population. Urban Ecosyst 21:10671074. https://doi.org/10.1007/s11252-018-0794-2
Shimamoto T, Uchida K, Koizumi I et al (2020) No evidence of physiological stress in an urban animal: comparison of fecal cortisol metabolites between urban and rural eurasian red squirrels. Ecol Res 35:243–251. https://doi.org/10.1111/1440-1703.12081
Shutt JD, Lees AC (2021) Killing with kindness: does widespread generalised provisioning of wildlife help or hinder biodiversity conservation efforts? Biol Conserv 261:109295. https://doi.org/10.1016/j.biocon.2021.109295
Suyama Y, Matsuki Y (2015) MIG-seq: an effective PCR-based method for genome-wide single-nucleotide polymorphism genotyping using the next-generation sequencing platform. Sci Rep 5:16963. https://doi.org/10.1038/srep16963
Takahata Y, Uchida K, Shimamoto T et al (2023) Supplemental feedings affect diet seasonality and niche width in urban eurasian red squirrels. J Mammal 104:1443–1454. https://doi.org/10.1093/jmammal/gyad089
Tsuji O, Yanagawa H, Muneoka T, Tsuchiya F (2004) Estimating of Russian flying squirrel habitat using GIS. (in Japanese). Jour JSIDRE 72:37–41
Tsuji O, Muneoka T, Takeda K, Tsuchiya F (2007) Estimation of windbreaks using GIS in Otofuke, Hokkaido. (in Japanese). J Jpn Soc Reveget Tech 32:404–411
Uchida K, Suzuki K, Shimamoto T et al (2016) Seasonal variation of flight initiation distance in eurasian red squirrels in urban versus rural habitat. J Zool 298:225–231. https://doi.org/10.1111/jzo.12306
Uchida K, Suzuki KK, Shimamoto T et al (2019) Decreased vigilance or habituation to humans? Mechanisms on increased boldness in urban animals. Behav Ecol 30:1583–1590. https://doi.org/10.1093/beheco/arz117
Uchida K, Yamazaki T, Ohkubo Y, Yanagawa H (2021) Do green park characteristics influence human-wildlife distance in arboreal squirrels? Urban Urban Green 58:126952. https://doi.org/10.1016/j.ufug.2020.126952
Wauters L, Dhondt A (1993) Immigration pattern and success in red squirrels. Behav Ecol Sociobiol 33:159–167. https://doi.org/10.1007/BF00216596
Wauters LA, Verbeylen G, Preatoni D et al (2010) Dispersal and habitat cuing of eurasian red squirrels in fragmented habitats. Popul Ecol 52:527–536. https://doi.org/10.1007/s10144-010-0203-z
Yanagawa H (2005) Traffic accidents involving the red squirrel and measures to prevent such accidents in Obihiro City, Hokkaido, Japan. Res Bull Obihiro Univ 25:35–37
Acknowledgements
We thank Hisashi Yanagawa, Iori Tanio, Ryunosuke Okawa, Shuki Kikuchi, and the Laboratory of Wildlife Ecology at Obihiro University of Agriculture and Veterinary Medicine for their support in conducting this field research. We thank Isturo Koizumi and Ayaka Sawada for providing the samples. We also thank the editor and anonymous reviewer for their constructive suggestions to improve the manuscript.
Funding
Open Access funding provided by Ritsumeikan University.
Author information
Authors and Affiliations
Contributions
All authors contribute to the study conception. Data were collected by Yu Takahata, Kenta Uchida, Tatsuki Shimamoto, and Yushin Asari. Data curation and analysis were performed by Yu Takahata and Yohey Terai. The first draft of the manuscript was written by Yu Takahata, Nobuyuki Kutsukake, and Yohey Terai, and all authors commented on previous version of the manuscript. All the authors have approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahata, Y., Uchida, K., Kutsukake, N. et al. Urbanisation has impacted the population genetic structure of the Eurasian red squirrel in Japan within a short period of 30 years. Conserv Genet (2024). https://doi.org/10.1007/s10592-024-01631-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10592-024-01631-9