Abstract
Introduced non-native species can threaten native species through interspecific hybridisation and genetic introgression. We assessed the prevalence of hybridisation and introgression between introduced European brown hare, Lepus europaeus, and the endemic Irish hare, L. timidus hibernicus. Roadkill hares (n = 56) were sequenced for a 379bp section of the mitochondrial DNA D-loop and a 474bp segment of the nuclear transferrin (Tf) gene. A species-specific indel in the transferrin gene was present in L.t. hibernicus and absent in L. europaeus. Excluding three hares from which molecular data could not be recovered, 28 hares (53%) were native L.t. hibernicus, 7 (13%) were non-native L. europaeus and 18 (34%) were hybrids; of which 5 (28%) were first generation (F1) involving bidirectional crosses with mismatched nuclear and mtDNA (3 ♂ europaeus x ♀ hibernicus and 2 ♂ hibernicus x ♀ europaeus). Mixed nuclear transferrin sequences suggested 13 (72%) of hybrids were at least 2nd generation (F2) with 9 (69%) possessing L.t. hibernicus and 4 (31%) L. europaeus mtDNA (the latter indicative of hybrid backcrossing with the non-native). The prevalence of hybridisation at similar mountain-brown hare contact zones throughout Europe is notably lower (4–16%) and typically unidirectional (♂ europaeus x ♀ timidus). A high prevalence of bidirectional hybridisation and introgression (in association with projected climate change) may favour the introduced species over the native. Genetic surveillance and population monitoring are needed to further explore the potential conservation implications of European brown hare in Ireland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In some circumstances, introduced non-native species can negatively impact native species most often due to competition for space and resources but sometimes by hybridisation (Rhymer and Simberloff 1996) and introgression; the backcrossing of hybrids with either parental species (Harrison and Larson 2014). Hybridisation may contribute to the success of an invader with an initially low population density as it reduces the Allee effect; the negative correlation between mean individual fitness and population size (Mesgarana et al. 2016). While hybrids are not always easy to identify from physical features, at the molecular level, hybridisation is clearly evidenced by introgression between species.
In conservation biology, hybridisation between introduced and native species is typically perceived as a threat to the native (Rosinger et al. 2021). Hybridisation and introgression can lead to genetic or demographic swamping by abundant hybrid individuals (Todesco et al. 2016). For example, non-native Sika deer, Cervus nippon, freely hybridise with native Red deer, Cervus elaphus, in Scotland and Ireland where hybrids can be more common than individuals with no hybrid ancestry (Abernethy 1994; Hayden and Harrington 2000; McDevitt et al. 2009; Smith et al. 2014). Similarly, the genetic integrity of the European wildcat, Felis sylvestris, is threatened due to introgression with hyperabundant domestic cats, F.s. domesticus (Nussberger et al. 2018). Native westslope cutthroat trout, Oncorhynchus clarkii lewisi, hybridise with introduced rainbow trout, Oncorhynchus mykiss, with first generation hybrids having a high reproductive success promoting introgression (Muhlfeld et al. 2009). In these cases and others, species range shifts due to climate change may increase interactions between introduced and native species driving further declines (Muhlfeld et al. 2014).Davis
In evolutionary biology, hybridisation can confer adaptive benefits improving the evolutionary success of hybrids. For example, obtaining maternally inherited mitochondrial DNA from another species whose metabolism is adapted to a different climatic zone can enhance local adaptation under a changing climate (Chunco 2014). Consequently, hybridisation occurs widely in some taxa, for example, hares, Lepus spp. (Ferreira et al. 2021), eroding genetic differences between diverging lineages driving reticulate evolution (Liu et al. 2011) and creating considerable taxonomic uncertainty (Thulin et al. 2006a, b; Melo-Ferreira et al. 2005, 2009, 2011; Alves et al. 2003, 2008a, b). For example, adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares, Lepus americanus, with brown winter coat colour (advantageous during the lower snowfall conditions) likely originated from an introgressed black-tailed jackrabbit, Lepus californicus, allele (Jones et al. 2018). The global rise in climate-change induced species range shifts and non-native species introductions, the intractability of their removal in most situations and equivocation over their impacts, have driven recent calls to stop demonising introduced species (Davis et al. 2021). Accepting the ecological change wrought by introduced species, and adopting a dispassionate evolutionary viewpoint, may lower the burden of interventionist conservation action but further biodiversity loss is to be expected in the immediate future (Simberloff 2011). As an example, the European brown hare, Lepus europaeus, was introduced to southern Sweden, where it hybridised and introgressed with the native mountain hare, the heath hare, L. timidus sylvaticus, replacing it and causing its virtual extinction in less than 200 years (Thulin 2003c).
The European brown hare has also been introduced to Ireland into the range of another endemic subspecies of mountain hare, the Irish hare, L. timidus hibernicus. It, along with the stoat, Mustela erminea hibernica (Martínková et al. 2007), grouse, Lagopus lagopus hibernicus (McMahon et al. 2012; Meyer-Lucht et al. 2016), dipper, Cinclus cinclus hibernicus (Hourlay et al. 2008), coal tit, Periparus ater hibernicus, and jay, Garrulus glandarius hibernicus, likely colonised Ireland naturally after the last glacial maximum and represent a community of genetically distinct endemic subspecies reflecting the island’s unique continental biogeography. The Irish hare exists on the westernmost fringe of the mountain hare’s distribution and differs phenotypically, behaviourally and ecologically from other mountain hares (Barrett Hamilton 1898; Dingerkus and Montgomery 2002, Angerbjörn and Flux 1995; Reid 2011). The Irish hare is genetically distinct from its closest geographic neighbours possessing a comparatively high number of unique mitochondrial haplotypes (Hughes et al. 2006; Hamill et al. 2006). The genetic composition of its population is consistent with the long-term accumulation of genetic differences due to local adaptation to snowless conditions (Giska et al. 2022) resulting from its persistence in ice free refuges during successive glacial advances in, or around, Ireland (Montgomery et al. 2014). Thus, hybridisation and introgression with introduced the European brown hare has at least the potential to threaten the Irish hare’s genetic integrity as an island endemic.
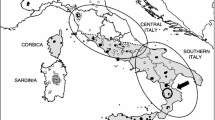
L. europaeus is one of a long line of mammalian introductions to Ireland by humans since the Holocene (Montgomery et al. 2014). Multiple deliberate introductions occurred throughout Ireland between 1848 and the 1890s (Reid 2011; Fig.1a). Barrett-Hamilton (1898) reported that several populations established successfully and proliferated, but most disappeared by the end of the 19th century. While there have been isolated reports of European brown hares (Fairley 2001; Sheppard 2004), the only confirmed extant population is 1,000–2,000 individuals in Mid-Ulster spanning south-east County Derry and east County Tyrone (Reid and Montgomery 2007; Caravaggi et al. 2015, 2016). Irish hares have adapted to warmer conditions than elsewhere in the mountain hare range, exploiting habitats from mountains to the coast (Lysaght and Marnell 2016) in the absence of a lowland competitor. Comparison of habitat niches suggest that L.t. hibernicus and L. europaeus have comparable niche breadths that may completely overlap (Caravaggi et al. 2017a). Both species show a preference for improved grassland over other habitats (Reid and Montgomery 2007). A long-term decline in L.t. hibernicus abundance was associated with climatic and agricultural change (Reid et al. 2021). Climate change models predict greater extremes of precipitation and warmer summers in Ireland in the late 21st century (Nolan and Flanagan 2020) making it more suitable for arable agriculture which may favour L. europaeus (Caravaggi et al. 2017a).
Here, we examine the genomic impact of L. europaeus on L.t. hibernicus in Ireland. L. europaeus may incorporate L.t. hibernicus mtDNA and nuclear DNA into its genome similar to L. europaeus with respect to native L. timidus in Sweden, the Alps and Russia (Zachos et al.2010; Thulin et al. 2003a-c 2006a,b) which may lead to elevated fitness of the introduced species. Persistent introgression and possible fixation of introgressed mtDNA and nuclear DNA establishing a hybrid lineage, could render the native species vulnerable to replacement. The fate of earlier introductions and the current status of European brown hares in Ireland, however, does not suggest that hybrids, if they exist, are undermining abundance of the native species. Alternatively, and perhaps more likely, introgressed nuclear and mtDNA genomes may be less fit and occur at reduced frequencies and disappear over subsequent generations as extant L. europaeus die out. We determine whether hybrids between L. europaeus and L.t. hibernicus exist in Ireland, estimate the prevalence of hybridisation, its directionality and the frequency of first and second generation hybrids and introgression, to assess the potential threat to the endemic Irish hare.
Methods
Tissue sampling
L.t. hibernicus is protected under the Wildlife and Natural Environment Act (Northern Ireland) 2011 (and its predecessors). Derogation under licence permits the taking of animals for scientific purposes, but systematic lethal sampling was deemed undesirable and incompatible with our conservation-orientated ethic. Thus, 56 roadkill hares were collected opportunistically from the vicinity of the non-native L. europaeus population established in Mid-Ulster (Counties Derry and Tyrone) accumulated between 2003 and 2008. Whilst sightings of L. europaeus have been made near Baronscourt Estate, west County Tyrone (Reid and Montgomery 2007), the population in Mid-Ulster is the only known extant population (Reid 2011; Caravaggi et al. 2016). No attempt was made to attribute a species phenotype to each carcass, which was determined genetically (see below), as many roadkill carcasses were badly damaged. An 8mm biopsy (Robbins Scientific Ltd.) was removed from the ear of each animal and preserved in 98% ethanol (Taggart et al. 1992).
Genetics
Taxonomic reliance solely on mtDNA may lead to erroneous conclusions, whilst a gene/marker in the nuclear genome that can accurately differentiate species based on unequivocal diagnostic sites, may be elusive (Alves et al. 2008b; Ben Slimon et al. 2008). Thus, to ensure reliable species identification, we used sequencing analysis of a 379 basepair (bp) segment of the mitochondrial DNA (mtDNA) D-loop, and a partial 474bp region, between exons 6 and 7 of the transferrin (tf) nuclear gene. Previous investigations (Hughes et al. 2006) revealed ≥ 32 fixed genetic differences (sequence mutations) between L.t. hibernicus and L. europaeus in the D-loop region. After careful examination of sequences of transferrin gene, we found a diagnostic site that could reliably discriminate L.t. hibernicus and L. europaeus, based on the presence/absence (indel) of a single species-specific nucleotide in the partial transferrin gene region. All L.t. hibernicus had a T at position 158bp while all L. europaeus had a deletion at this position. This diagnostic site was reported as valuable in assessing phylogenetic relationships of L. europaeus and other hare species by Alves et al. (2003, 2008a,b). We examined the relationship between transferrin haplotypes from L. europaeus samples from Ireland (this study), L. europaeus and L. timidus from Europe including those available from Genbank, and L.t. hibernicus from across Ireland beyond the non-native range of L. europaeus (from Hughes et al. 2006), to determine the reliability of the species diagnostic characters in transferrin.
The mtDNA D-loop and nuclear tf gene were amplified using polymerase chain reaction (PCR) primers using conditions described in Wallner et al. (2001). Amplified PCR products were purified using Microspin columns (©Roche) and were bidirectionally sequenced (Macrogen Inc.). Resulting sequences for both mtDNA and nuclear regions were checked and assembled using the software packages Chromas v2.1 (Technelysium Pty Ltd.) and BioEdit v7.0.5.3 Multiple Sequence Editor (Hall 1999).
Discordant introgressions in nuclear and mitochondrial genomes are indicative of the direction of hybridisation i.e. assigning male and female parentage to species. The phylogenetic analysis based on separate mtDNA fragments were generally concordant. For nuclear DNA data, each individual bidirectional sequence and each polymorphic position in the dataset was carefully scanned to ensure that all double peaks were correctly identified and consistently scored across samples. We used offset chromatogram peaks to directly infer the phase of some or all heterozygous positions in those individuals that were heterozygous for an indel.
Results
All but one of 42 L. europaeus examined had a deletion in the nuclear transferrin gene. The sole exception was from Alava, Northern Spain. Thus, the presence of a T residue at 157bp as opposed to a deletion, is likely diagnostic of L. timidus and L. europaeus, respectively. Either mitochondrial or nuclear sequences could not be identified reliably in three hares (Supplementary Information, Table S1) which were excluded from subsequent analyses leaving n = 53. Twenty-eight hares (53%) were identified as endemic L.t. hibernicus and 7 (13%) were non-native L. europaeus with each possessing its own species-specific mtDNA and nuclear markers (Fig.1b; Table S1). There were also 18 putative hybrids (34% of identified samples) which possessed a combination of mtDNA from one species and nuclear DNA or mixed nuclear sequences from the another. Of these hybrids, 5 (28%) were first generation (F1) involving bidirectional crosses with mismatched nuclear and mtDNA i.e. 3 ♂ europaeus x ♀ hibernicus and 2 ♂ hibernicus x ♀ europaeus (Fig.1b; Table S1). Mixed nuclear transferrin sequences suggested 13 hares (72% of hybrids and 25% of all hares) were at least 2nd generation (F2) of which 9 (50% of hybrids) had hibernicus and 4 (22% of hybrids) had europaeus mtDNA (the former indicative of hybrid backcrossing with the native and the latter indicative of backcrossing with the non-native i.e. bidirectional introgression). The generational timing of such backcrossing was undetermined.
(a) Known L. europaeus introduction sites (brown circles) in Ireland (showing county boundaries for orientation) as reported by Barrett-Hamilton (1898) though all died out except those at Baronscourt Estate, west Tyrone. Subsequently, sightings (tan 10km squares) were reported in north Donegal (Fairley 2001; Sheppard 2004) and Mid-Ulster spanning south-east Derry and east Tyrone (Caravaggi et al. 2016). (b) Infographic showing 53 specimens from Mid-Ulster identified using species-specific mitochondrial (bottom lane) and nuclear (top lane) DNA as L.t. hibernicus (green) or L. europaeus (brown) or mixed sequences (hatched green/brown) and species ID (top). (c) Distribution of genetically identified samples relative to the non-native range of L. europaeus as surveyed during 2012-13 (Caravaggi et al. 2016) showing named urban areas (grey) for orientation. Pie charts show the percentage of specimens at each location which were native (green), non-native (brown) or hybrids (hatched green/brown with bold boundary) with charts scaled to represent sample sizes (1 < n < 15). Hare thumbnails show L.t hibernicus (left ©Mike Brown), L. europaeus (right ©Mark Hamblin) and a europaeus x timidus hybrid during winter (middle ©Mark Hamblin)
Hybrids spanned an area 40km north to south along the eastern range edge margin of L. europaeus down the western shore of Lough Neagh (Fig.1c). Both L. europaeus and hybrids were found up to 13km south of the former’s known southerly range edge margin suggesting it may be more widespread than recorded by Caravaggi et al. (2016) with genomic impacts well beyond its known range.
Screening of an additional 24 L.t. hibernicus sampled throughout Ireland beyond the immediate vicinity of the range of L. europaeus (> 15km) failed to provide any evidence of hybridisation or introgression suggesting known failed historical introductions elsewhere left no lasting genomic impact. The 12 hybrids from Mid-Ulster possessing L.t. hibernicus mtDNA D-loop haplotypes shared these with the additional 24 L.t. hibernicus sampled throughout Ireland.
Discussion
Molecular markers and species ID
Sequencing mtDNA (D-loop) and a portion of the nuclear gene transferrin (tf) revealed a deletion in the L. europaeus transferrin sequence that differentiated them from L.t. hibernicus. This deletion in the L. europaeus transferrin sequence not present in L. timidus, has been reported previously (Melo-Ferriera et al. 2009). With the exception of one out of 42 individuals, all L. europaeus had a deletion at this site, supporting this as a diagnostic marker for this species. The exception was a specimen from Alava, Northern Spain, a region with previous evidence of ancient and recent mitochondrial and nuclear introgression (Melo-Ferreira et al. 2009). Thus, it is likely that this L. europaeus acquired the T polymorphism from L. timidus.
Hybridisation in Ireland
A mismatch between species-specific mitochondrial and nuclear DNA indicated animals had hybrid ancestry. At contact zones between L. timidus and L. europaeus throughout Europe, hybridisation is typically unidirectional between female L. timidus and male L. europaeus (Alves et al. 2003; Thulin et al. 2003a-c, Melo-Ferreira et al. 2005; Zachos et al. 2010) which are larger and more likely to engage in mate guarding behaviour than male L. timidus (Flux and Angerbjorn 1990). In Ireland, we detected bidirectional, first generation (F1) hybrids with three examples of the former direction and two examples of male L.t. hibernicus mating with female L. europaeus. Despite a small sample size, it seems that bidirectional crosses occurred at a similar frequency (17% and 11% of hybrids respectively). Irish hares (3.5–4.5kg) are the largest of the 16 subspecies of L. timidus (others being 2.5–3.5kg typically; Angerbjörn and Flux 1995) and may compete more effectively with L. europaeus males than their smaller relatives, thus evening up the odds for successful hybrid mating in either direction. L. timidus male x L. europaeus female crosses have been achieved by artificial insemination in captivity in Sweden (Gustavsson and Sundt 1965), but such hybrids (and their descendants) may suffer a competitive disadvantage in the wild (Thulin and Tegelström 2002).
Bidirectional introgression was also recorded in the present study with hybrids backcrossing predominately with the native (69% of individuals with mixed nuclear sequences had L.t. hibernicus mtDNA) with L. europaeus backcrossing more limited: 31% of individuals with mixed nuclear sequences had L.t. hibernicus mtDNA). This > 2:1 ratio reflects the 3:1 non-native:native ratio in population density at the invading wavefront of L. europaeus (Caravaggi et al. 2016) suggesting the frequency of bidirectional introgression reflects the populations of parental and hybrid stock and availability of potential mates.
Notwithstanding any potential skew or bias due to our opportunistic sampling strategy (and associated small sample size), the prevalence of hybridisation and introgression in Ireland at 33% of sampled individuals was notably higher than (unidirectional hybridisation and introgression) reported from Sweden at 7–16% (Thulin and Tegelstrom 2002; Jansson et al. 2007), and Russia and Switzerland, both at 4%, where L. europaeus is expanding its range (Thulin et al. 2006c; Zachos et al. 2010). Bidirectional hybridisation and introgression has also been reported at much lower frequencies than in Ireland (present study), in Russia (Thulin et al. 2006a), Scandinavia (Thulin et al. 2006b; Melo-Ferreira et al. 2009) and the Alps (Suchentrunk et al. 2005, 2006; Melo-Ferreira et al. 2009, 2011; Zachos et al. 2010).
Irish hares screened throughout Ireland beyond the range of L. europaeus revealed no evidence of any L. europaeus genomic legacy beyond their current range. Hughes et al. (2006) examined > 100 hares for mtDNA and screened > 1,100 hares throughout Ireland for microsatellite markers exhibiting variation in allelic frequency between L. timidus and L. europaeus (OCMSAT5, SAT5 and IH270) but found no evidence consistent with the presence of L. europaeus. Thus, despite sightings (Dingerkus 1997; Sheppard 2004), and notwithstanding unknown populations, most historical L. europaeus introductions to Ireland appear to have died out without trace except for those reported here, in Northern Ireland, within which hybridisation and introgression with the endemic Irish hare is extensive. This suggests that the future of L. timidus hibernicus may not be threatened genetically by the presence of introduced L. europaeus under prevailing conditions without substantial expansion of the non-native’s population. Speculatively, introgression has the potential to convey adaptive potential, for example, L. europaeus hybrids could acquire mountain hare mitochondrial DNA enhancing metabolism such as a homeostatic ability to tolerate cool, damp conditions typical throughout Ireland while L.t. hibernicus hybrids could acquire the European brown hare’s tolerance for higher temperatures potentially mitigating the impacts of climate change.
Lepus hybridisation and introgression
Hybridisation and introgression are ubiquitous throughout the genus Lepus and throughout their distribution and involve ancient as well as recent phenomena (Alves et al. 2003; Liu et al. 2011; Acevedo et al. 2012; Melo-Ferreira et al. 2014a, b; Marques et al. 2017a; Seixas et al. 2018; Ashrafzadeh et al. 2018; Momhammadi et al. 2020). Phylogenetic or deep introgression is associated with species replacements during glacial advances and retreats (Melo-Ferreira et al. 2014c; Kinoshita et al. 2019). More recent introgression occurs where there are parapatric distributions, perhaps leading to a hybrid zone, or species expansions due to climate change or introductions (Zachos et al. 2010; Cheng et al. 2014; Schenker et al. 2020). Deep introgression can result in a gradient in introgression which is a record of species replacement and a similar phenomenon is apparent in expanding populations. In both scenarios, the level of introgression is highest close to the invasion wavefront (Marques et al. 2017a). Introgression in hares mainly involves mtDNA and is generally unidirectional (Melo-Ferreira et al. 2012, 2014a) but there are exceptions to both generalisations (Wu et al. 2011; Levanen et al. 2018a, b). Whilst phylogenetic studies produce bifurcating trees, the level of introgression in past and current relationships amongst hare lineages is best represented by a network referred to as reticulate evolution e.g. Liu et al. (2011), Acevedo et al. (2015) and Tolesa et al. (2017).
Introgression in hares may be selected for and is adaptive: for example, camouflage in snowshoe hares Lepus americanus (Jones et al. 2018), coat colour variants (Giska et al. 2019, 2022); structural and physicochemical properties of proteins encoded by the OXHPOS complex in Arctic lineages of Northern American hares (Melo-Ferreira et al. 2014b); and genes associated with spermatogenesis, immunity and mitochondrial metabolism in northern populations of L. granatensis (Seixas et al. 2018). Thus, hares are excellent models for research on the interplay of biogeography and evolution where there are marked discontinuities in global change due to climate change and anthropogenic introductions (Acevedo et al. 2012; Marques 2017b; Reid et al. 2021).
Future of european brown hare in Ireland
The current restricted geographical distribution of L. europaeus in Ireland suggests that it has not spread far from its point of introduction in Mid-ulster and that its origin may be as recent as the 1970s (Caravaggi et al. 2016). L.t. hibernicus possesses a high level of genetic diversity being isolated in Ireland for a longer period of time and adapted to a wider range of lowland and upland conditions than L. timidus subspecies in Scandinavia and the Alps (Hughes et al. 2006; Giska et al. 2022). As such, L. t. hibernicus may be a stronger ecological competitor for L. europaeus than its continental relatives. Thus, the outcome for this population may follow the pattern of earlier introductions of L. europaeus throughout Ireland becoming extinct leaving little or no genetic trace. However, it is still possible that the high degree of hybridisation and introgression described here in conjunction with climate change or some other environmental factor(s) may result in a more persistent interaction and a long-lasting genomic legacy.
Model simulations suggest that if an invasion is recent and interbreeding events are infrequent, introgression of genes tends to be from the native to the invader irrespective of relative densities of the two, and symmetrical (bidirectional), but becomes asymmetric (unidirectional) with increasing interbreeding levels (Currat et al. 2008). This is consistent with empirical data with 32 cases (82%) of invasions and range expansion showing this pattern (Currat et al. 2008). In Sweden, most of the asymmetric transfer of L. timidus mtDNA into L. europaeus may have occurred during the initial rapid expansion of the invader with hybrid frequency declining 10–20 years following introduction, indicating that interspecies mating occurs when there is a lack of conspecific partners. Thus, initial impact on the genetic make-up of the species is worse at the beginning of the invasion but should decline over time. Hybrid occurrence was higher in areas of current sympatry with 9 of 12 L. europaeus carrying L. timidus mtDNA at the wavefront of the invasion compared to 11 of 70 in former areas of sympatry (Jansson et al. 2007). This process did not apparently impact Swedish L. timidus genetically, but caused L. timidus to retreat northwards with the near extinction of the heath hare, L.t. sylvaticus, whose range was restricted to south Sweden (Thulin 2003c). Swedish L. timidus are limited by resources in the lowland grasslands in the south while the northward expansion of L. europaeus is constrained by the snowline (Jansson et al. 2007).
In Ireland, bidirectional hybridisation and symmetrical transfer of genetic material may become more asymmetric, L. europaeus absorbing L.t. hibernicus mtDNA, as the invasion progresses, and transfer of nuclear L.t. hibernicus DNA to L. europaeus becoming less likely. If both species remain at low densities, L. europaeus may simply die out in common with previous introductions. Nevertheless, the L. europaeus population has expanded in Ireland since its first discovery perhaps indicating a growing propensity for persistence. Certainly, future change in climate (and likely associated changes in agriculture) in Ireland are likely to favour the invader and disadvantage the native (Caravaggi et al. 2017a).
Nineteenth century densities of L.t. hibernicus were probably much higher and widely adapted to the habitat in Ireland (Reid et al. 2021). However, L.t. hibernicus numbers declined dramatically during the early 20th century due to habitat fragmentation and landscape homogenisation due to agricultural intensification, and climatic change (Reid et al. 2021), to reach current low densities (McGowan et al. 2019). Early introductions of L. europaeus during the mid- to late-1800s, therefore, may have been met with greater resistance than the more recent introduction to Mid-Ulster where low densities of the native offer little resistance to invasion. Hybrid hares in Sweden and Russia occur where densities of each species are low, and, hence, frequency dependent, assortative mating can arise (Thulin et al. 2006a, b; Jansson et al. 2007). This can occur when one species dominates the other or when both species occur at low density allowing the two species to hybridise due to low availability of conspecific partners, known as Hubbs’ principle (Chan and Levin 2005). L.t. hibernicus occurs at around 3 hares/km2 in allopatry, L. europaeus in their core range are up to 5 hares/km2, with densities of both species roughly equal in sympatry (Caravaggi et al. 2016). The balance of competition between both species likely pivots on subtle differences in habitat choice with the L.t. hibernicus preferring pastoral agriculture and L. europaeus preferring areas with a higher coverage of arable crop.
Monitoring, surveillance and sampling
In the Republic of Ireland, L. timidus is listed on Annex V of the EU Habitats and Species Directive 92/43/EEC with Articles 11 and 17 requiring regular monitoring and reporting on its conservation status while monitoring and surveillance, management and eradication of non-native species like L. europaeus is covered by the EU Regulation 1143/2014 on Invasive Alien Species. Northern Ireland is no longer an EU member after the United Kingdom left the EU in 2021 with the impact of legislative transition on conservation priorities unclear. Regardless, being one of Ireland’s few truly endemic mammals (Montgomery et al. 2014), any potential threat to the status of L.t. hibernicus should be taken very seriously by Government agencies and Departments in both jurisdictions north and south of the border. A European brown hare invasive Species Action Plan (iSAP) is required galvanising stakeholders (Government, landowners, farmers and the public) to support monitoring and surveillance of hare distribution and abundance and systematic sampling of hares to evaluate genomic impacts. The attitudes of landowners and others are important in setting an agenda for conservation of Irish hare such that a program of outreach and engagement may be required to attain majority support for lethal control of invading L. europaeus (Caravaggi et al. 2017b). Notwithstanding calls for eradication, this interspecific interaction zone offers an excellent study system by which to test ideas and concepts involved in invasion biology, population dynamics and evolutionary biology.
Data Availability
The datasets generated are available from the corresponding author on reasonable request.
References
Abernethy K (1994) The establishment of a hybrid zone between red and sika deer (genus Cervus). Mol Ecol 3:551–562
Acevedo P, Jimenez-Valverde A, Melo-Ferreira J, Real R, Alves PC (2012) Parapatric species and the implications for climate change studies: a case study on hares in Europe. Glob Change Biol 18:1509–1519
Acevedo P, Melo-Ferreira J, Farelo L, Beltran-Beck B, Real R, Campos R, Alves PC (2015) Range dynamics driven by Quaternary climate oscillations explain the distribution of introgressed mtDNA of Lepus timidus origin in hares from the Iberian Peninsula. J Biogeog 42:1727–1735
Alves PC, Ferrand N, Suchentrunk F, Harris DJ (2003) Ancient introgression of Lepus timidus mtDNA into L. granatensis and L. europaeus in the Iberian Peninsula. Mol Phylo Evol 27:70–80
Alves PC, Melo-Ferreira J, Freitas H, Boursot P (2008a) The ubiquitous mountain hare mitochondria: multiple introgressive hybridisation in hares, genus Lepus. Phil Trans R Soc B 363:2831–2839
Alves PC, Melo-Ferreira J, Branco M, Suchentrunk F, Ferrand N, Harris DJ (2008b) Evidence for genetic similarity of two allopatric European hares (Lepus corsicanus and L. castroviejoi) inferred from nuclear DNA sequences. Mol Phylo Evol 46:1191–1197
Angerbjörn A, Flux JEC (1995) Lepus timidus Mamm Species 495:1–11
Ashrafzadeh MR, Djan M, Szendrei L, Paulauskas A, Scandura M, Bagi Z, Ilie DE, Kerdikoshvili N, Marek P, Soos N, Kusza S (2018) Large-scale mitochondrial DNA analysis reveals new light on the phylogeography of Central and Eastern-European Brown hare (Lepus europaeus Pallas, 1778. PLoS ONE 13:10: e0204653
Barrett-Hamilton GEH (1898) Notes on the introduction of the brown hare into Ireland. Ir Nat J 7:69–76
Ben Slimen H, Suchentrunk F, Elgaaied ABA (2008) On shortcomings of using mtDNA sequence divergence for the systematics of hares (genus Lepus): An example from cape hares. Mamm Biol 73:25–32
Caravaggi A, Montgomery WI, Reid N (2015) Range expansion and comparative habitat use of insular, congeneric lagomorphs: invasive European hares Lepus europaeus and endemic Irish hares Lepus timidus hibernicus. Biol Invasions 18:1217–1218
Caravaggi A, Zaccaroni M, Riga F, Schai-Braun SC, Dick JTA, Montgomery WI, Reid N (2016) An invasive-native mammalian species replacement process captured by camera trap survey random encounter models. Rem Sens Ecol Conserv 2:45–58
Caravaggi A, Leach K, Santilli F, Rintala J, Helle P, Tiainen J, Bisi F, Martinoli A, Montgomery WI, Reid N (2017a) Niche overlap of mountain hare subspecies and the vulnerability of their ranges to invasion by the European hare; the (bad) luck of the Irish. Biol Invasions 19(2):655–674
Caravaggi A, Montgomery WI, Reid N (2017b) Management and control of invasive brown hares (Lepus europaeus): contrasting attitudes of selected environmental stakeholders and the wider rural community. Biol Environment-Proc Roy Ir Acad 117B:53–63
Chan KM, Levin SA (2005) Leaky prezygotic isolation and porous genomes: rapid introgression of maternally inherited DNA. Evolution 59:720–729
Cheng E, Hodges KE, Melo-Ferreira J, Alves PC, Mills LS (2014) Conservation implications of the evolutionary history and genetic diversity hotspots of the snowshoe hare. Mol Ecol 23:2929–2942
Chunco AJ (2014) Hybridization in a warmer world. Ecol Evol 4:2019–2031
Currat M, Ruedi M, Petit RJ, Excoffier L (2008) The hidden side of invasions: massive introgression by local genes. Evol Int J Org Evol 62:1908–1920
Davis M, Chew M, Hobbs R et al (2021) Don’t judge species on their origins. Nature 474:153–154
Dingerkus SK (1997) The distribution and ecology of the Irish Hare L. t. hibernicus in Northern Ireland. Unpubl PhD Thesis. The Queen’s University of Belfast, Belfast, UK
Dingerkus SK, Montgomery WI (2002) A review of the status and decline in abundance of the Irish hare (Lepus timidus hibernicus) in Northern Ireland. Mamm Rev 32:1–11
Fairley J (2001) A basket of weasels. Belfast. Privately published
Ferreira M, Jones M, Callahan C, Farelo L, Tolesa Z, Suchentrunk F, Boursot P, Mills S, Alves P, Good J, Melo-Ferreira J (2021) The legacy of recurrent introgression during the radiation of hares. Syst Biol 70:593–607
Flux JEC, Angerbjorn R (1990) The hares and jackrabbits. In: Chapman JA, Flux JEC (eds) Rabbits, hares and pikas; status survey and action plan. IUCN Switzerland, Gland, pp 61–94
Giska I, Farelo L, Pimenta J, Seixas FA, Ferreira MS, Marques JP, Miranda I, Letty J, Jenny H, Hacklander K, Magnussen E, Melo-Ferreira J (2019) Introgression drives repeated evolution of winter coat color polymorphism in hares. Proc Natl Acad Sci USA 116:24150–24156
Giska I, Pimenta J, Farelo L, Boursot P, Hackländer K, Jenny H, Reid N, Montgomery WI, Prodöhl PA, Alves PC, Melo-Ferreira J (2022) The evolutionary pathways for local adaptation in mountain hares. Mol Ecol 31:1487–1503
Gustavsson I, Sundt CO (1965) Anwendung von kunstlicher Befruchtung bei der Hybridiserung von zwei Hasenarten. Z Jagdwiss 11:155–158
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hamill RM, Doyle D, Duke EJ (2006) Spatial patterns of genetic diversity across European subspecies of mountain hare, Lepus timidus L. Heredity 1–11
Harrison RG, Larson EL (2014) Hybridization, introgression, and the nature of species boundaries. J Hered 105(S1):795–809
Hayden T, Harrington R (2000) Exploring Irish Mammals. Townhouse Dublin, Ireland
Hourlay F, Libois R, D’Amico F, Sara M, O’Halloran J, Michaux JR (2008) Evidence of a highly complex phylogeographic structure on a specialist river bird species, the dipper (Cinclus cinclus). Mol Phylogenet Evol 49:435–444
Hughes MF, Montgomery WI, Prodohl PA (2006) Population genetic structure and systematics of the Irish hare. Unpubl report prepared by Quercus for the Environment and Heritage Service, DOE NI
Jansson G, Thulin C-J, Pehrson à (2007) Factors related to the occurrence of hybrids between brown hares (Lepus europaeus) and mountain hares (L. timidus in Sweden). Ecography 30:709715
Jones MR, Mills LS, Alves PC, Callahan CM, Alves JM, Lafferty DJR, Jiggins FM, Jensen JD, Melo-Ferreira J, Good JM (2018) Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares. Science 360:1355–1358
Kinoshita G, Nunome M, Kryukov AP, Kartavtseva IV, Han SH, Yamada F, Suzuki H (2019) Contrasting phylogeographic histories between the continent and islands of East Asia: Massive mitochondrial introgression and long-term isolation of hares (Lagomorpha: Lepus). Mol Phylogenet Evol 136:65–75
Levanen R, Kunnasranta M, Pohjoismaki J (2018a) Mitochondrial DNA introgression at the northern edge of the brown hare (Lepus europaeus) range. Ann Zool Fenn 55:15–24
Levanen R, Thulin CG, Spong G, Pohjoismaki JLO (2018b) Widespread introgression of mountain hare genes into Fennoscandian brown hare populations.PLOS ONE13 Article Number: e0191790
Liu J, Yu L, Arnold ML, Wu CH, Wu SF, Lu X, Zhang YP (2011) Reticulate evolution: frequent introgressive hybridization among Chinese hares (genus Lepus) revealed by analyses of multiple mitochondrial and nuclear DNA loci.BMC Evol Biol11 Article Number:223
Lysaght L, Marnell F (eds) (2016) Atlas of Mammals in Ireland 2010–2015. National Biodiversity Data Centre, Waterford
Marques JP, Farelo L, Vilela J, Vanderpool D, Alves PC, Good JM, Boursot P, Melo-Ferreira J (2017a) Range expansion underlies historical introgressive hybridization in the Iberian hare.Sci Rep7 Article Number:40788
Marques JP, Ferreira MS, Farelo L, Callahan CM, Hacklander K, Jenny H, Montgomery WI, Reid N, Good JM, Alves PC, Melo-Ferreira J (2017b) Data Descriptor: Mountain hare transcriptome and diagnostic markers as resources to monitor hybridization with European hares. Sci Data 4 Article Number: 70178
Martínková N, McDonald RA, Searle JB (2007) Stoats (Mustela erminea) provide evidence of natural overland colonisation of Ireland. Proc. Roy. Soc. B 274: 387e1393
McDevitt AD, Edwards CJ, O’Toole P, O’Sullivan P, O’Reilly C, Carden RF (2009) Genetic structure of, and hybridisation between, red (Cervus elaphus) and sika (Cervus nippon) deer in Ireland. Mamm Biol 74:263–273
McGowan N, McDermott N, Stone R, Lysaght L, Dingerkus K, Caravaggi A, Kerr I, Reid N (2019) National hare survey & population assessment 2017-19. Irish Wildlife Manual, vol 113. National Parks & Wildlife Service, Dublin, Ireland
McMahon BJ, Johansson MP, Piertney SB, Buckley K, Höglund J (2012) Genetic variation among endangered Irish red grouse (Lagopus lagopus hibernicus) populations: implications for conservation and management. Conserv Genet 13:639–647
Melo-Ferreira J, Boursot P, Suchentrunk F, Ferrand N, Alves PC (2005) Invasion from the cold past: extensive introgression of mountain hare (Lepus timidus) mitochondrial DNA into three other hare species in northern Iberia. Mol Ecol 14:2459–2464
Melo-Ferreira J, Alves PC, Freitas H, Ferrand N, Boursot P (2009) The genomic legacy from the extinct Lepus timidus to the three hare species of Iberia; contrast between mtDNA, sex chromosomes and autosomes. Mol Ecol 18:2643–2658
Melo-Ferreira J, Alves PC, Rocha J, Ferrand N, Boursot P (2011) Interspecific x-chromosome and mitochondrial DNA introgression in the Iberian hare; selection or allele surfing? Evolution 65:1956–1968
Melo-Ferreira J, Boursot P, Carneiro M, Esteves PJ, Farelo L, Alves PC (2012) Recurrent Introgression of Mitochondrial DNA Among Hares (Lepus spp.) Revealed by Species-Tree Inference and Coalescent Simulations. Syst Biol 61:367–381
Melo-Ferreira J, Farelo L, Freitas H, Suchentrunk F, Boursot P, Alves PC (2014a) Home-loving boreal hare mitochondria survived several invasions in Iberia: the relative roles of recurrent hybridisation and allele surfing. Heredity 112:265–273
Melo-Ferreira J, Seixas FA, Cheng E, Mills LS, Alves PC (2014b) The hidden history of the snowshoe hare, Lepus americanus: extensive mitochondrial DNA introgression inferred from multilocus genetic variation. Mol Ecol 23:4617–4630
Melo-Ferreira J, Vilela J, Fonseca MM, da Fonseca RR, Boursot P, Alves PC (2014c) The elusive nature of adaptive mitochondrial DNA evolution of an Arctic lineage prone to frequent introgression. Genome Biol Evol 6:886–896
Mesgarana MB, Lewis MA, Ades PK, Donohoe K, Ohadi DS, Li C, Cousins RD (2016) Hybridization can facilitate species invasions, even without enhancing local adaptation. Proc Natl Acad Sci USA 113:10210–10214
Meyer-Lucht Y, Mulder KP, James MC, McMahon BJ, Buckley K, Piertney SB, Höglund J (2016) Adaptive and neutral genetic differentiation among Scottish and endangered Irish red grouse (Lagopus lagopus scotica). Conserv Genet 17:615–630
Montgomery WI, Provan J, McCabe AM, Yalden DW (2014) Origin of British and Irish mammals: disparate post-glacial colonisation and species introductions. Quat Sci Rev 98:144–165
Muhlfeld CC, Kalinowski ST, McMahon TE, Taper ML, Painter S, Leary RF, Allendorf FW (2009) Hybridization rapidly reduces fitness of a native trout in the wild. Biol Lett 5:328–331
Muhlfeld CC, Kovach RP, Jones LA, Al-Chokhachy R, Boyer MC, Leary RF, Lowe WH, Luikart G, Allendorf FW (2014) Invasive hybridization in a threatened species is accelerated by climate change. Nat Clim Chang 4:620–624. DOI: https://doi.org/10.1038/NCLIMATE2252
Nolan P, Flanagan J (2020) High-Resolution Climate Projections for Ireland-A Multi-Model Ensemble Approach. Environmental Protection Agency. https://www.epa.ie/pubs/reports/research/climate/researchrepor
Nussberger B, Currat M, Quilodran CS, Ponta N, Keller LF (2018) Range expansion as an explanation for introgression in European wildcats. Biol Conserv 218:49–56
Reid N, Montgomery WI (2007) Is the naturalisation of the brown hare in Ireland a threat to the endemic Irish hare? Biol Environ-Proc Roy Ir Acad 107B3:129–138
Reid N (2011) European hare (Lepus europaeus) invasion ecology; implication for the conservation of the endemic Irish hare (Lepus timidus hibernicus). Biol Invasions 13:559–569
Reid N, Brommer JE, Stenseth NC, Marnell F, McDonald RA, Montgomery WI (2021) Regime shift tipping point in hare population collapse associated with climatic and agricultural change during the very early 20th century. Glob Change Biol 27:3732–3740
Rosinger HS, Geraldes AM, Nurkowski KA, Battlay P, Cousens RD, Rieseberg LH, Hodgins KA (2021) The tip of the iceberg: Genome wide marker analysis reveals hidden hybridization during invasion. Mol Ecol 30:810–825
Rhymer JM, Simberloff D (1996) Extinction by hybridisation and introgression. Ann Rev Ecol Syst 27:83–109
Schenker L, Bollmann K, Rehnus M, Brodbeck S, Gugerli F (2020) Hare’s affairs: Lessons learnt from a noninvasive genetic monitoring for tracking mountain hare individuals. Ecol Evol 10:10150–10166
Sheppard R (2004) Brown hares Lepus europaeus Pallas in N. W. Ireland. Ir Nat J 2712:484–485
Seixas FA, Boursot P, Melo-Ferreira J (2018) The genomic impact of historical hybridization with massive mitochondria DNA introgression.Genome Biol19 Article Number: 91
Simberloff D Non-natives: 141 scientists object.Nature 475, 36
Smith SL, Carden RF, Coad B, Birkitt T, Pemberton JM (2014) A survey of the hybridisation status of Cervus deer species on the island of Ireland. Conserv Genet 15:823–835
Suchentrunk F, Mamuris Z, Stamatis C (2005) Introgressive hybridisation in wild living mountain hare (L. timidus varronis) and brown hares (L. europaeus) and morphological consequences. Mamm Biol 70(supplement):39–40
Suchentrunk F, Ben Slimen H, Stamatis C, Sert H, Scandura M, Apollinio M, Mamuris Z (2006) Molecular approaches revealing prehistoric, historic, or recent translocations and introductions of hare (Genus: Lepus) by humans. Hum Evol 21:151–165
Taggart JB, Hynes RA, Prodöhl PA, Ferguson A (1992) A simplified protocol for routine total DNA isolation from salmonid fishes. J Fish Biol 40:963–965
Thulin C-G, Tegelström H (2002) Biased geographical distribution of mitochondrial DNA that passed the species barrier from mountain hares to brown hares (genus Lepus): an effect of genetic incompatibility and mating behaviour? J Zool 258:299–306
Thulin C-G, Jaarola M, Tegelström H (2003a) The occurrence of mountain hare mitochondrial DNA in wild brown hares. Mol Ecol 6(5):463–467
Thulin C-G, Tegelström H, Fredga K (2003b) Haplotype diversity of mountain hare mtDNA among native mountain hares and introduced brown hares in Scandinavia. Ann Zool Fenn 40:45–52
Thulin C-G (2003c) The distribution of mountain hares Lepus timidus in Europe: a challenge from brown hares L. europaeus? Mammal Rev 33(1):29–42
Thulin C-G, Stone J, Tegelström H, Walker CW (2006a) Species assignment and hybrid identification among Scandinavian hares Lepus europaeus and L. timidus. Wildl Biol 12:29–38
Thulin C-G, Fang M, Averianov AO (2006b) Introgression from Lepus europaeus to L. timidus in Russia revealed by mitochondrial single nucleotide polymorphisms and nuclear microsatellites. Hereditas 143:68–76
Todesco M, Pascual MA, Owens GL, Ostevik KL, Moyers BT, Hubner S, Heredia SM, Hahn MA, Caseys C, Bock DG, Rieseberg LH (2016) Hybridization and extinction. Evol Appl 9 7 SI 892–908
Tolesa Z, Bekele E, Tesfaye K, Ben Slimen H, Valqui J, Getahun A, Hartl GB, Suchentrunk F (2017) Mitochondrial and nuclear DNA reveals reticulate evolution in hares (Lepus spp., Lagomorpha, Mammalia) from Ethiopia. PLOS ONE12 Article Number:e0180137
Wallner B, Huber S, Achmann R (2001) Non-invasive PCR sexing of rabbits (Oryctolagus cuniculus) and hares (Lepus europaeus). Mamm Biol 66:190–192
Wu YH, Xia L, Zhang Q, Yang QS, Meng XX (2011) Bidirectional introgressive hybridization between Lepus capensis and Lepus yarkandensis. Mol Phylogenet Evol 59:545–555
Zachos FE, Ben Slimen H, Hackländer K, Guacometti M, Suchentrunk F (2010) Regional genetic in situ differentiation despite phylogenetic heterogeneity in Alpine mountain hares. J Zool 282:47–53
Acknowledgements
Dr Declan Looney was the Northern Ireland Environment Agency (NIEA) Client Officer. We thank Ciaran McLarnon, NIEA from Peatlands Park, Co. Armagh for collecting specimens as well as: Declan Coney, Patrick Dennison, Stephen Foster, Colin Gates, Patrick Gernan, Gill Henderson, Pol McCann Michael Miles, Walter Mills, E. Mulholland, Maeve Rafferty and Mike Rendle. We also thank Dr. Rebecca Smith, Bristol University and the British Association for Shooting and Conservation (BASC) for donating European brown hare specimens from England.
Funding
This project was funded by the Northern Ireland Environment Agency (NIEA) through the Natural Heritage Research Partnership (NHRP) with Quercus, Queen’s University Belfast (QUB).
Author information
Authors and Affiliations
Contributions
N.R. identified presence of European brown hares in Mid-Ulster, verified their history and absence elsewhere in Ireland, collected samples, drafted the manuscript including production of the figures and is the corresponding author. M.F.H collected samples, extracted DNA and conducted genetic analyses. R.A.H. developed molecular markers and supervised laboratory training and analyses. W.I.M. secured funding and contributed intellectually throughout. P.P. supervised genetic work and contributed intellectually throughout. N.R., W.I.M and P.P. edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reid, N., Hughes, M.F., Hynes, R.A. et al. Bidirectional hybridisation and introgression between introduced European brown hare, Lepus europaeus and the endemic Irish hare, L. timidus hibernicus. Conserv Genet 23, 1053–1062 (2022). https://doi.org/10.1007/s10592-022-01471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-022-01471-5