Abstract
Histopathological Growth Patterns (HGPs) have prognostic and predictive value in patients with Colorectal Liver Metastases (CRLM). This study examined whether preoperative measurement of Circulating Tumour Cells (CTCs) is associated with HGP. CTCs were prospectively enumerated in 7.5 ml of blood using the FDA-approved CellSearch system in patients who underwent local treatment of CRLM with curative intent between 2008 and 2021. All CTC samples were collected on the day of local treatment. Patients treated with neoadjuvant chemotherapy for CRLM or with extrahepatic disease at the time of CTC sampling were excluded. HGP was scored retrospectively following the current consensus guidelines. The association between CTCs and HGP was investigated through multivariable logistic regression. Data were available for 177 patients, desmoplastic HGP (dHGP) was observed in 34 patients (19%). There were no statistically significant differences in patient and tumour characteristics between dHGP and non-dHGP at baseline. Patients with dHGP had longer overall – and disease-free survival (logrank p = 0.003 and 0.003, respectively) compared to patients with non-dHGP. CTCs were not detected in 25(74%) of dHGP patients and in 68(48%) of non-dHGP patients (chi-squared p = 0.006). Preoperative absence of CTCs was the only significant predictor for dHGP in multivariable logistic regression (Odds Ratio 2.7, 95%CI 1.1–6.8, p = 0.028), Table 3. Preoperative absence of CTCs is associated with dHGP in chemo naive CRLM patients without extrahepatic disease. Based on our results, CTC count alone is not sufficient to preoperatively identify HGPs, but integration of CTC count in multivariable prediction models may aid the preoperative identification of HGPs of CRLM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histopathological growth patterns (HGPs) are a prognostic biomarker in patients who have undergone a resection of colorectal cancer liver metastases (CRLM) [1,2,3]. The evaluation of HGPs is standardized in international consensus guidelines [1, 4]. The assessment of HGPs is reliable, accurate and is conducted on routine Hematoxylin & Eosin (H&E) slides of resected CRLM that are already available for all patients who have undergone resection for CRLM [5]. HGPs can be classified into biologically distinct subtypes: desmoplastic HGP (dHGP) and non-desmoplastic HGP (non-dHGP) [4, 6]. Patients with pure dHGP have favourable Overall- (OS) and Disease Free Survival (DFS) compared to patients with any presence of non-dHGP in the resected tumor [2,3,4]. The prognostic value of HGPs is independent of other known prognostic factors like Fong clinical risk score [7] and KRAS mutation status [2, 3]. Aside from the prognostic value, recent studies suggest that HGPs are associated with the effectiveness of adjuvant systemic chemotherapy in patients with CRLM [8, 9].
Preoperative knowledge of HGP could provide useful information to guide individual treatment plans, for example to select patients for neoadjuvant therapy. However, preoperative assessment of HGPs is not possible and postoperative pathologic examination of resected metastases is currently the only method to assess HGP of liver metastases [4].
Prediction of HGP through a surrogate marker such as Circulating Tumour Cells (CTCs) may be helpful to integrate HGP in preoperative decision making without the need for upfront pathologic examination. CTCs are a prognostic biomarker in various malignant tumours. It remains unclear whether the presence of CTCs is associated with poor OS and DFS in patients with CRLM [10,11,12,13].
The aim of this study was to investigate whether measurement of preoperative CTCs could predict HGPs of resected CRLM.
Methods
Study design and patients
A retrospective single center analysis was conducted to explore the association between CTCs and HGP. To include as many patients as possible in this study, patients were selected from two datasets from previous prospective studies on CTCs in patients with CRLM. All patients have undergone curative intent local treatment (i.e. all preoperatively identified lesions were treated) at the Erasmus Medical Center, Rotterdam, the Netherlands between January 2008 and December 2021. All patients had a pathologically confirmed primary colorectal tumour. An overview of both datasets is provided in supplementary table 1. All patients from dataset 1 underwent venous blood sampling and all patients from dataset 2 underwent arterial blood sampling.
Patients were excluded if they had undergone neo-adjuvant chemotherapy for CRLM since neoadjuvant chemotherapy may influence HGP assessment and CTC enumeration [14,15,16]. Patients were also excluded if extrahepatic disease was present at the time of CTC sampling.
Assessments
Per patient 30 mL blood was collected in CellSave tubes and assessed for CTCs at a local Erasmus MC laboratory. All study samples were collected preoperatively on the day of local treatment of CRLM. Samples were processed within 24 h of collection. The 30 mL whole blood samples were reduced to 7.5 mL enriched blood samples by ficoll density gradient separation, which has been described previously [17, 18]. CTCs were enumerated using the CELLSEARCH CTC kit (Menarini Silicon Biosystems, Castel Maggiore, Italy). CTC enumeration results were reviewed by 2 trained operators [13].
The CELLSEARCH CTC kit contains magnetic beads coated with anti-epithelial-cell adhesion molecule antibodies in order to imunomagnetically enrich epithelial cells from whole blood. The remaning cells are stained with DAPI, anti CK 8, 18 or 19 antibodies and anti CD45 antibodies. The sample is then transferred to a Magnest Cell Preservation Device, after which the cells are scanned by the Cell Spotter Analyzer, which is a four-color semi-automated fluorescence microscope. The images are assessed by trained readers. CTCs are selected based on the following criteria: size ≥ 4 µm, round to oval morphology, positive staining for CK8, 18 or 19, a visible DAPI positive nucleus, at least 50% overlap between nucleus and cytoplasm and negative staining for CD45.
HGPs were scored retrospectively on H&E slides of liver metastases following the current consensus guidelines [1, 4]. HGPs were scored per H&E slide as a percentage of the total tumour-liver interface on all available H&E slides for each patient. The average HGP was subsequently calculated per lesion and (in case of multiple lesions) per patient to yield a single HGP score for each patient [1, 4]. Based on recent studies, the cutoff for dHGP was set at pure dHGP (100%) versus any amount of non-dHGP [2,3,4]. Pure dHGP will be referred to as “dHGP” and any amount of non-dHGP will be referred to as “non-dHGP” for the remainder of this paper.
Fong clinical risk score was calculated for all patients and classified as low (< 3 points) and high risk (≥ 3 points) [7].
Statistics
Baseline characteristics for dHGP and non-dHGP, as well as for the two datasets were compared using fischer’s exact test for discrete variables and the Kruskal–Wallis test for continuous variables. Continuous variables are represented as median (95% CI) unless indicated otherwise. OS and DFS of the HGPs and the OS and DFS of detectable and non-detectable CTCs were assessed via Kaplan–Meier analysis and compared using the log-rank test. OS was defined as the time in months from resection of CRLM to death of any cause. DFS was defined as the time in months from resection of CRLM to recurrence of disease or death. The prognostic value of CTCs was evaluated through multivariable cox regression analysis. The association between CTCs and HGP was evaluated through multivariable logistic regression. Patient and tumour characteristics were evaluated through univariable logistic regression. Predictors with a p < 0.2 were included in multivariable logistic regression and subsequently excluded via backward elimination. Number of CRLM was included in multivariable analysis to correct for tumour load. A p-value of < 0.05 was considered statistically significant.
Results
A total of 177 patients were included who underwent surgery between January 2008 and December 2021. The baseline characteristics are shown in Table 1. No statistically significant differences in patient and tumour characteristics were observed between dHGP and non-dHGP patients at baseline. There were 34 patients with dHGP (19%). Five-year OS in the dHGP patients was 86% (95% CI 73–100%) compared to 44% (95%CI 35–56%) in non-dHGP patients (logrank p = 0.003). Five-year DFS was 39% (95% CI 23–65%) in the dHGP patients compared to 19% (95% CI 13%-28%) in non-dHGP patients, p = 0.003.
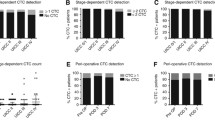
There was a statistically significant difference in CTC counts between the HGP groups. The median CTC count for dHGP was 0.0 (IQR0.0–0.8), the median CTC count for non-dHGP was 1.0 (IQR 0.0–2.0), p = 0.034. The distribution of the CTC counts per HGP groups is shown in Fig. 1.
Table 2 shows the baseline characteristics per CTC category. The group with detectable CTCs contained more male patients than the group without detectable CTCs, 68 (73%) compared to 49 (58%), p = 0.038. There were more patients with metastasis > 5 cm in diameter in the detectable CTC group than in the group without detectable CTCs, 20 patients (24%) versus 4 patients (4%) respectively, p < 0.001. No other statistically significant differences were observed between these groups. Five-year OS was 39% (95% CI 30–50%) for patients with detectable CTCs and 57% (95%CI 49–66%) for patients without detectable CTCs (p = 0.065). DFS was 23% (95% CI 16–34%) for detectable CTCs and 27% (95% CI 20–36%) for non-detectable CTCs, p = 0.879. CTC status (detectable/non-detectable) was not associated with OS (HR 0.8, 95% CI 0.5–1.3, p = 0.35) or DFS (HR 95%CI 1.0, 0.7–1.5, p = 0.84) in multivariable analysis. The multivariable analysis corrected for the following known predictors of survival in patients with CRLM: HGP, location of the primary colorectal tumour, lymph node status of the primary tumour, disease free interval between resection of the primary tumour and detection of CRLM, number of CRLM, diameter of the largest CRLM, CEA before liver resection. Significant predictors for OS in multivariable analysis were: dHGP (HR 0.45, 95% CI 0.22–0.89, p = 0.02), left-sided primary tumour (HR 0.47, 95%CI 0.24–0.92, p = 0.03) and diameter of the largest CRLM > 5 cm (HR 2.41, 95%CI 1.16–5.02. p = 0.02). Significant predictors for DFS in multivariable analysis were: dHGP (HR 0.45 95%CI 0.26–0.77), Disease Free Interval < 12 months (HR 1.74 95%CI 1.15–2.36, p < 0.01) and > 1 CRLM (HR 1.83 95%CI 1.22–2.74, p < 0.01). Kaplan–Meier curves as well as the full Cox regression analysis are provided as supplementary Fig. 1 and supplementary table 2, respectively.
CTCs were not detected in 74% (n = 25) of patients with dHGP and in 48% of patients (n = 68) with non-dHGP (p = 0.006). There were no statistically significant differences in these proportions between arterial and venous blood samples. An overview of the CTC counts is provided in supplementary table 3.
The sensitivity of absent CTCs for dHGP was 74%, the specificity of absent CTCs for dHGP was 58%. The positive predictive value of absent CTCs for dHGP was 27%, the negative predictive value of absent CTCs for dHGP was 89%.
The absence of CTCs remained the only significant predictor for dHGP in multivariable logistic regression with an odds ratio 2.7 (95%CI 1.1–6.8; p = 0.028). The uni-and multivariable results are shown in Table 3.
Discussion
In this retrospective study including 177 patients who underwent liver resection for CRLM an association was found between HGPs and CTCs. In multivariable logistic regression, the absence of CTCs was associated with presence of dHGP. However, CTCs were absent in 74% of patients with dHGP compared to 48% of non-dHGP. Since non-dHGP is more common than dHGP, the measurement of CTCs alone is therefore not sufficient to predict HGP preoperatively to use it in a clinical setting. Given the association with HGP, CTCs may be useful as a factor in multivariable preoperative prediction models for HGP. There are several potential factors that may be predictive for HGP. Recent studies suggest that the histopathology of the primary colorectal tumour may be correlated to HGPs of CRLM [19, 20]. Studies have also shown promising results in predicting HGP based on preoperative imaging using radiomics and artificial intelligence [21, 22]. Combining the predictors above could result in a model that may be accurate enough to use in preoperative decision making.
HGPs are a promising biomarker in patients with CRLM, which has several potential advantages for clinical use. The evaluation of HGPs is standardized in international consensus guidelines [1, 4]. The assessment of HGPs is reliable, accurate and is conducted on routine H&E slides of resected CRLM that are available for all patients who have undergone resection for CRLM [5]. HGPs are a strong predictor of OS and DFS after curative intent resection of CRLM and are independent of other known prognostic factors like Fong clinical risk score[7] and KRAS mutation status [2, 3, 23]. In addition, there is evidence to suggest that HGPs may also have predictive value in patients with CRLM [8, 9]. A disadvantage of HGPs is that they can only be scored postoperatively on slides of resected CRLM, making them unavailable until resection has taken place. Preoperative assessment of HGP would allow clinicians to fully utilize the prognostic and predictive capabilities of HGP [8]. Prediction of HGPs using a minimally invasive method would enable preoperative the use of HGPs.
CTCs may be a prognostic marker for OS and DFS in patients with CRLM [10, 24, 25]. The presence of CTCs has been associated with worse outcomes, although the most informative cutoff remains unclear [26]. CTCs may also be predictive in patients who receive chemotherapy for metastatic colorectal cancer [27]. To our knowledge, this is the first study investigating the association between the phenotype of CRLM and CTCs.
No association between CTC counts and OS or DFS was found in the current study. However, no conclusions on this subject can be drawn from this data as the retrospective study design, with different methods of CTC sampling does not lend itself well to answer this research question. The study may not have the statistical power to detect clinically relevant differences in OS or DFS between different CTC groups with statistical significance. A prospective, multicenter Dutch study evaluating the association between CTCs and DFS has almost completed the follow-up [28].
The difference in the proportion of patients with non-detectable CTCs between dHGP and non-dHGP patients in this study has yet to be explained. Previous studies have shown an association between tumour burden and CTC counts in patients with CRLM [29]. In the current study the group with detectable CTCs had significantly larger metastases. There was no significant difference in tumour burden between dHGP and non-dHGP, there was even a trend towards more liver metastases in dHGP compared to non-dHGP. Moreover, a statistically significant association remained between HGPs and CTCs in multivariable analysis when correcting for diameter and number of liver metastases. Tumour burden appears to offer no explanation for the differences in CTC status between dHGP and non-dHGP found in this study. There is evidence that patients with non-dHGP have a higher risk of extrahepatic- and multi-organ recurrence after treatment of CRLM [30]. The higher proportion of patients with extrahepatic disease and the higher proportion of detectable CTCs in patients with non-dHGP compared to dHGP may be the result of a shared underlying mechanism. Previous studies on the tumour microenvironment of the different HGPs in CRLM have shown an increased immune infiltrate, enriched with cytotoxic CD8 + T-cells in dHGP when compared to non-dHGP [31]. The association between immune infiltrate including CD8 + T-cells with prognosis has been shown for both primary and metastatic colorectal cancer [32,33,34,35,36] and suggests more anti-tumour immune activity in dHGP, which may contribute to the favourable OS and DFS for dHGP compared to non-dHGP [2, 3, 31]. Similarly, an effective immune response may affect CTC counts, where evasion of immune surveillance is proposed as one of the major contributors to the presence of CTCs in the circulation [37, 38]. In summary, liver metastases with pure dHGP are associated with an increased immune response in the liver[31] and the associated lack of CTCs signifies anti-tumour immunity in dHGP. However, the significance of our finding needs to be validated after which causality can be explored.
A limitation of the study is the combination of two separate datasets for the analysis. An important difference between the two datasets that were used is that the CTCs were enumerated in venous blood in the 86 patients of dataset 1 and in arterial blood in the 91 patients of dataset 2. Previous studies have suggested that arterial blood samples may be superior to detect CTCs compared to venous samples, [39, 40] even though arterial samples may be more challenging to collect in clinical practice. In the current study, we found no statistically significant difference in median CTC count between both datasets. In addition, the percentages of detectable CTCs between dHGP and non-dHGP for both sampling methods were similar. Given the similarities in CTC counts, HGP proportions, and overall patient and tumour characteristics between both cohorts, it is unlikely that the use of two cohorts has compromised the current study.
Another limitation of this study is external validity. To relate HGP of liver metastases to CTCs, patients with extrahepatic disease were excluded to prevent measurement of CTCs from other metastatic locations than CRLM. Patients receiving neo-adjuvant chemotherapy were excluded as well, because previous studies have shown that neo-adjuvant chemotherapy may alter the HGPs of CRLM [14], and neo-adjuvant chemotherapy may also influence the detection of CTCs [15, 16]. This selection may be appropriate for the current research question, but the strict in- and exclusion criteria result in a population that may not fully resemble the current clinical practice. For instance, most patients in this study had favorable tumour characteristics and a low tumour load. The majority (70%) had a low Fong Clinical Risk score [7].
The lack of genetic mutation data is another limitation of this study. KRAS, BRAF and MSI status is not routinely evaluated in Dutch clinical practice, leading to this data being unavailable for most patients of the cohort. Primary tumour location was used as a covariate in multivariable analysis, which may somewhat mitigate the lack of mutation data as right sided primary tumours are correlated with increased incidence of genetic mutations [41].
In conclusion, in this study the absence of preoperative CTC was associated with dHGP in patients with colorectal liver metastases, however, CTCs alone are insufficient for preoperative prediction of HGP for use in clinical practice. Based on our results CTC enumeration could represent a valuable addition to preoperative prediction models for HGP.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
van Dam PJ et al (2017) International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer 117(10):1427–41. https://doi.org/10.1038/bjc.2017.334
Galjart B et al (2019) Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis 22(2):355–68. https://doi.org/10.1007/s10456-019-09661-5
Hoppener DJ et al (2021) Histopathological growth patterns and survival after resection of colorectal liver metastasis: an external validation study. JNCI Cancer Spectr 5(3):pkab026. https://doi.org/10.1093/jncics/pkab026
Latacz E et al (2022) Histopathological growth patterns of liver metastasis: updated consensus guidelines for pattern scoring, perspectives and recent mechanistic insights. Br J Cancer. https://doi.org/10.1038/s41416-022-01859-7
Hoppener DJ et al (2019) Histopathological growth patterns of colorectal liver metastasis exhibit little heterogeneity and can be determined with a high diagnostic accuracy. Clin Exp Metastasis 36(4):311–9. https://doi.org/10.1007/s10585-019-09975-0
Vermeulen PB et al (2001) Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol 195(3):336–42. https://doi.org/10.1002/path.966
Fong Y et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3):309–18. https://doi.org/10.1097/00000658-199909000-00004 (discussion 18-21)
Buisman FE et al (2020) Histopathological growth patterns as biomarker for adjuvant systemic chemotherapy in patients with resected colorectal liver metastases. Clin Exp Metastasis 37(5):593–605. https://doi.org/10.1007/s10585-020-10048-w
Frentzas S et al (2016) Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 22(11):1294–302. https://doi.org/10.1038/nm.4197
Arrazubi V et al (2019) Circulating tumor cells in patients undergoing resection of colorectal cancer liver metastases. Clinical utility for long-term outcome: a prospective trial. Ann Surg Oncol 26(9):2805–11. https://doi.org/10.1245/s10434-019-07503-8
Brudvik KW et al (2015) Detection of circulating tumor cells at surgery and at follow-up assessment to predict survival after two-stage liver resection of colorectal liver metastases. Ann Surg Oncol 22(12):4029–37. https://doi.org/10.1245/s10434-015-4482-7
Rossi GF et al (1978) Discussion on the causes of failure of surgical treatment of partial epilepsies. Appl Neurophysiol 41(1–4):29–37. https://doi.org/10.1159/000102397
Lalmahomed ZS et al (2015) Prognostic value of circulating tumour cells for early recurrence after resection of colorectal liver metastases. Br J Cancer 112(3):556–61. https://doi.org/10.1038/bjc.2014.651
Nierop PM et al (2022) Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases. J Pathol Clin Res 8(1):48–64. https://doi.org/10.1002/cjp2.235
Lorente D et al (2016) Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. Eur Urol 70(6):985–92. https://doi.org/10.1016/j.eururo.2016.05.023
Bidard FC et al (2014) Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15(4):406–14. https://doi.org/10.1016/S1470-2045(14)70069-5
Lalmahomed ZS et al (2010) Circulating tumor cells and sample size: the more, the better. J Clin Oncol 28(17):e288-9. https://doi.org/10.1200/JCO.2010.28.2764
Gross S, et al. (2005) Modified Ficoll preprocessing procedure for 30 mL of whole blood prior to CellSearch circulating tumor cell test. Presentation at the 5th International Symposium on Minimal Residual Cancer
Abe H et al (2022) Histological growth patterns of colorectal cancer liver metastases: a strong prognostic marker associated with invasive patterns of the primary tumor and p53 alteration. Hum Pathol 123:74–83. https://doi.org/10.1016/j.humpath.2022.02.015
Wu JB et al (2019) Histologic features and genomic alterations of primary colorectal adenocarcinoma predict growth patterns of liver metastasis. World J Gastroenterol 25(26):3408–25. https://doi.org/10.3748/wjg.v25.i26.3408
Granata V et al (2022) Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of colorectal liver metastases growth pattern. Diagnostics (Basel). https://doi.org/10.3390/diagnostics12051115
Starmans MPA et al (2021) Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis 38(5):483–94. https://doi.org/10.1007/s10585-021-10119-6
Buisman FE et al (2022) Predicting 10-year survival after resection of colorectal liver metastases; an international study including biomarkers and perioperative treatment. Eur J Cancer 168:25–33. https://doi.org/10.1016/j.ejca.2022.01.012
Seeberg LT et al (2015) Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg 261(1):164–71. https://doi.org/10.1097/SLA.0000000000000580
Groot Koerkamp B et al (2013) Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol 20(7):2156–65. https://doi.org/10.1245/s10434-013-2907-8
Gazzaniga P et al (2013) Circulating tumor cells in metastatic colorectal cancer: do we need an alternative cutoff? J Cancer Res Clin Oncol 139(8):1411–6. https://doi.org/10.1007/s00432-013-1450-0
Sastre J et al (2012) Circulating tumor cell count is a prognostic factor in metastatic colorectal cancer patients receiving first-line chemotherapy plus bevacizumab: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. Oncologist 17(7):947–55. https://doi.org/10.1634/theoncologist.2012-0048
Institute EMC (2015) Research project | Liquid biopsies in colorectal liver metastasis patients: MIRACLE. Erasmus MC Cancer Institute, Rotterdam
Kaifi JT et al (2015) Circulating tumor cell levels are elevated in colorectal cancer patients with high tumor burden in the liver. Cancer Biol Ther 16(5):690–8. https://doi.org/10.1080/15384047.2015.1026508
Nierop PMH et al (2019) Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis 36(2):109–18. https://doi.org/10.1007/s10585-019-09960-7
Hoppener DJ et al (2020) Enrichment of the tumour immune microenvironment in patients with desmoplastic colorectal liver metastasis. Br J Cancer 123(2):196–206. https://doi.org/10.1038/s41416-020-0881-z
Ohtani H (2007) Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun 7:4
Sideras K et al (2018) Prognostic value of intra-tumoral CD8(+) /FoxP3(+) lymphocyte ratio in patients with resected colorectal cancer liver metastasis. J Surg Oncol 118(1):68–76. https://doi.org/10.1002/jso.25091
Diederichsen AC et al (2003) Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother 52(7):423–8. https://doi.org/10.1007/s00262-003-0388-5
Glaire MA et al (2019) Tumour-infiltrating CD8(+) lymphocytes and colorectal cancer recurrence by tumour and nodal stage. Br J Cancer 121(6):474–82. https://doi.org/10.1038/s41416-019-0540-4
Lazarus J et al (2019) Mathematical modeling of the metastatic colorectal cancer microenvironment defines the importance of cytotoxic lymphocyte infiltration and presence of PD-L1 on antigen presenting cells. Ann Surg Oncol 26(9):2821–30. https://doi.org/10.1245/s10434-019-07508-3
Wang WC et al (2018) Survival mechanisms and influence factors of circulating tumor cells. Biomed Res Int 2018:6304701. https://doi.org/10.1155/2018/6304701
Ward MP et al (2021) Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell? Mol Cancer 20(1):59. https://doi.org/10.1186/s12943-021-01347-1
Terai M, Mu Z, Eschelman DJ, Gonsalves CF, Kageyama K, Chervoneva I, Orloff M, Weight R, Mastrangelo MJ, Cristofanilli M, Sato T (2015) Arterial blood, rather than venous blood, is a better source for circulating melanoma cells. EBioMedicine 2(11):1821-6. https://doi.org/10.1016/j.ebiom.2015.09.019
Fang ZT et al (2014) Circulating tumor cells in the central and peripheral venous compartment—assessing hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Onco Targets Ther 7:1311–1318. https://doi.org/10.2147/OTT.S62605ott-7-1311
Stintzing S et al (2017) Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer 84:69–80. https://doi.org/10.1016/j.ejca.2017.07.016
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Authorship contributions Conception and design of the study: DG, PV, CV, SS Acquisition of data: YM, SW, JK, PV Analysis and interpretation of data: YM, PO, SW, JM Manuscript drafting: YM, PO Manuscript revision: YM, PO, SW, JK, PV, JM, DG, SS, CV.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meyer, Y.M., Wilting, S.M., Kraan, J. et al. Circulating tumour cells are associated with histopathological growth patterns of colorectal cancer liver metastases. Clin Exp Metastasis 40, 69–77 (2023). https://doi.org/10.1007/s10585-022-10191-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-022-10191-6