Abstract
Natural selection alters the distribution of phenotypes as animals adjust their behaviour and physiology to environmental change. We have little understanding of the magnitude and direction of environmental filtering of phenotypes, and therefore how species might adapt to future climate, as trait selection under future conditions is challenging to study. Here, we test whether climate stressors drive shifts in the frequency distribution of behavioural and physiological phenotypic traits (17 fish species) at natural analogues of climate change (CO2 vents and warming hotspots) and controlled laboratory analogues (mesocosms and aquaria). We discovered that fish from natural populations (4 out of 6 species) narrowed their phenotypic distribution towards behaviourally bolder individuals as oceans acidify, representing loss of shyer phenotypes. In contrast, ocean warming drove both a loss (2/11 species) and gain (2/11 species) of bolder phenotypes in natural and laboratory conditions. The phenotypic variance within populations was reduced at CO2 vents and warming hotspots compared to control conditions, but this pattern was absent from laboratory systems. Fishes that experienced bolder behaviour generally showed increased densities in the wild. Yet, phenotypic alterations did not affect body condition, as all 17 species generally maintained their physiological homeostasis (measured across 5 different traits). Boldness is a highly heritable trait that is related to both loss (increased mortality risk) and gain (increased growth, reproduction) of fitness. Hence, climate conditions that mediate the relative occurrence of shy and bold phenotypes may reshape the strength of species interactions and consequently alter fish population and community dynamics in a future ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The increasing emissions of anthropogenic CO2 into the atmosphere are rapidly changing the physico-chemical conditions of the world’s oceans by increasing their acidity and surface temperatures (Caldeira and Wickett 2003; IPCC 2013). Ocean acidification and warming are set to challenge marine life by modifying their physiology and behaviour (Nagelkerken and Connell 2015) leading to altered biodiversity and ecosystem health (Bellard et al. 2012; Nagelkerken et al. 2017; Wittmann and Pörtner 2013; Connell et al. 2018). Organisms may be able to persist environmental change by shifting their ranges, (epi)genetic adaptation, and adaptive phenotypic plasticity (Nunney 2016; Souza et al. 2018). The persistence of sessile organisms with limited dispersal capacity will depend more heavily on phenotypic plasticity, as they cannot readily move towards more favourable environments as climate becomes unfavourable (Valladares et al. 2007; Reed et al. 2011; Leung et al. 2019). Phenotypic plasticity is the capacity of a single genotype to express multiple phenotypes in response to environmental stimuli (Scheiner 1993; Pigliucci 2005; Souza et al. 2018) allowing organisms to cope with environmental change (Bonamour et al. 2019). The capacity to improve an individual’s fitness to altered conditions is offered by phenotypic plasticity, a suite of adaptive responses that can bolster population persistence (Schmid and Guillaume 2017; Bonamour et al. 2019). Alternatively, plasticity can be maladaptive if fitness is reduced, or neutral if there is no effect on fitness (Ghalambor et al. 2007). We currently know little about how phenotypic plasticity and its variance across species and ecosystems might allow marine vertebrates to acclimate under climate change stressors, as most empirical studies have focussed on phenotypic means instead of their variability (O’Dea et al. 2019). It is also unclear whether such plasticity is sufficient to allow their populations to persist under future conditions.
Plastic responses in an individual’s morphological, physiological, and behavioural traits are a fundamental source of variation in a population (Henn et al. 2018; Gibert and Brassil 2014; Matesanz et al. 2010; Sultan and Spencer 2002). Behavioural traits such as activity, swimming levels, and boldness have implications for feeding and growth rates (Biro et al. 2003; Laubenstein et al. 2018). These traits also affect survivorship as they mediate exposure and susceptibility to predators (Biro et al. 2003; White et al. 2013). Bold individuals are characterised by increased risk-taking behaviours, which may provide them with increased access to resources but also increase their predation risk. Boldness is regulated by various physiological and biochemical processes, and because it is a heritable trait it is subject to natural selection (Ferrari et al. 2016).
In natural systems, selection fluctuates in space and time (Buskirk 2017) and favours specific phenotypes over others, i.e. those that are better pre-adapted to the novel conditions (Edelaar et al. 2017). A single phenotype cannot maintain fitness across a wide range of environments; therefore, selection across heterogeneous environments might favour plasticity that promotes diversification of traits (Reed et al. 2011; Lafuente and Beldade 2019). Populations can undergo three patterns of natural selection. The first one is directional selection where selection acts towards a single phenotypic extreme, shifting the distribution to one end (Kingsolver and Pfennig 2007). When selection acts in one direction and there is a lack of phenotypic variability, the vulnerability of these populations increases (Assis et al. 2016). A second mode of selection is stabilising selection, where fitness increases for individuals closest to the mean value (Kingsolver and Pfenning 2007). A third mode of selection is disruptive selection, where the highest levels of fitness are found at the extremes of the trait values (Kingsolver and Pfenning 2007).
Species responses to climate change are typically expressed as the mean value of their traits, disregarding the fact that population variability in phenotypes can modify the patterns of species interactions and natural selection (Gibert and Brassil 2014; Bolnick et al. 2011). Understanding the changes in the direction, frequency, or variability of the frequency distribution of phenotypes can indicate whether a population will be able to persist in a future climate. Whether a specific phenotype will be selected depends on the adaptive capacity of specific phenotypic traits to the changing environment. While abiotic conditions influence the selection of species or populations with particular traits and phenotypes that aid them to establish, persist, and reproduce (environmental filtering), biotic interactions can also be a significant contributor (Kraft et al. 2015; Lozada-Gobilard et al. 2019). Phenotype selection can modify demographic parameters that alter population sizes. Populations that undergo alterations in size and phenotypic distribution tend to experience altered interactions with other species populations (Donelson et al. 2019). Consequently, modified species interactions have the capacity to alter the structure of community in fluctuating environments (Nagelkerken and Munday 2016).
Here, we test the hypothesis that under future climate the frequency distribution of physiological and behavioural phenotypic traits in fishes shifts from present-day conditions, altering the overall phenotypic variability and affecting species population sizes. We investigate how the phenotypic distribution of different behavioural and physiological trait responses within populations of various fish species adjusts to future climate, simulated under natural and laboratory conditions. We used natural volcanic CO2 vents to test for effects of elevated CO2, and natural climate-warming regions to test for the effects of elevated temperature. Laboratory evaluations of future climate effects were performed using mesocosm and aquarium systems. A wide range of behavioural and physiological traits in 17 fish species (commonly found at the collection sites) were quantified to study resultant changes in trait frequency distributions within species populations. Assessing which phenotypes predominate in a changing ocean provides an understanding of their potential persistence or vulnerability under global change.
2 Materials and methods
2.1 Natural s ystems
2.1.1 Natural CO 2 seeps
This study was conducted on a temperate rocky reef at White Island, a volcanic island in Bay of Plenty, New Zealand (during 2013, and 2015–2018). Sample sites were located along the north-eastern coast of the island and consisted of two independent vent sites (north and south) and two independent controls sites (north and south) (see Fig. S1 in Connell et al. 2018). The two vent sites represented future CO2-enriched oceans for the year 2100 (approximate RCP 4.5–6.0 scenarios, Bopp et al. 2013) without confounding differences in water temperature, were located at 6–8-m water depth, and had a dimension of ~ 24 × 20 m each (Supplementary methods). The control sites represented current ambient pH levels and were situated ~ 25 m away from the vent sites. Studies undertaken over multiple time points showed that the seawater chemistry (pH, pCO2 values) is relatively consistent over time at the study sites (Nagelkerken et al. 2021). Temperature levels did not differ between vent (mean across years: 20.48 °C, table S1) and control (20.45 °C) sites. Vents were characterised by a benthic community dominated by turf algae (< 10 cm in height), and the control sites comprised a mosaic of sparse kelp (Ecklonia radiata), turf macroalgae, and hard-substratum sea urchin barrens devoid of vegetation (Connell et al. 2018).
2.1.2 Test of anti-predator behaviour
At natural CO2 vents, species can move in and out of the vent areas, reducing their exposure to elevated CO2. Hence, antipredator behaviour was evaluated for the most common site-attached species of fish at the study site, which are continuously exposed to elevated levels of CO2 at the vents due to their small home ranges of ~ 2 m2 (Syms and Jones 1999): common triplefin Forsterygion lapillum, crested blenny Parablennius laticlavius, Yaldwin’s triplefin Notoclinops yaldwyni, blue-eyed triplefin Notoclinops segmentatus, variable triplefin Forsterygion varium, and the scaly damselfish Parma alboscapularis (Supplementary methods). Antipredator responses were quantified at controls and at the CO2 vents by simulating the approach of a potential threat to the fish (a metal ruler) while video recording their escape behaviour, and measuring the startle distance at which fish initiated a flight response (see details in Nagelkerken et al. 2016) (Supplementary methods). In situ observations have shown that this closely mimics the startle distance to the approach of natural predators (Nagelkerken et al. 2017). All experiments were performed under animal ethics approval no. S-015-019 by the University of Adelaide Animal Ethics Committee.
2.1.3 Natural warming hotspots
The study sites were located along the coast of southeast Australia, which is considered a hotspot for ocean warming (Poloczanska et al. 2007; Figueira et al. 2009), where a latitudinal temperature gradient occurs (spatial increase towards lower latitudes) with accelerated warming occurring at the higher latitudes (temporal increase with time) (Figueira et al. 2009). Fish were sampled during the summer of 2017 and 2018 at different sites across these latitudes to represent colder or warmer regions: two sites in South West Rocks; two sites in Port Stephens (warm region, 25.6 °C average summer temperature); four sites in Sydney (either a warm or cold region, depending on the fish affinity: warm for mado (Atypichthys strigatus) vs cold for brown tang (Acanthurus nigrofuscus) and convict tang (Acanthurus triostegus); 23 °C average summer temperature); and six sites distributed between Narooma and Merimbula (cold region, 20.1 °C average summer temperature). Samples were taken during summer when there is a consistent peak in recruitment of tropical fish species. The collected tropical species are considered vagrants and occur mainly during the summer months at the sampled sites (Booth et al. 2007).
2.1.4 Test of anti-predator behaviour
Antipredator behaviour was tested using the same device and method as for the natural CO2 vents. These tests included juveniles of five fish species: the coral reef–associated species Acanthurus nigrofuscus (brown tang), Acanthurus triostegus (convict tang), and Abudefduf vaigiensis (Indo-Pacific sergeant), and the temperate species Atypichthys strigatus (mado) and Microcanthus strigatus (stripey) (Supplementary methods). The former three species are common range-extending coral reef fishes (Booth et al. 2018). All experiments were performed under animal ethics approval no. S-2017-002 (University of Adelaide) and ETH17-1117 (University of Technology Sydney) and followed the University’s animal ethics guidelines
2.2 Laboratory systems
A combination of large multi-species mesocosms and small single-species aquaria was used to test for behavioural responses in laboratory settings.
2.2.1 Mesocosm experimental design
Juvenile fishes were collected using a seine net along different coastal sites in the northern part of the Spencer Gulf and the eastern coast of the Gulf St. Vincent, South Australia, from September to October 2016. Three pelagic species, small-mouthed hardyhead (Atherinosoma microstoma), gold spot mullet (Liza argentea), yellow-eyed mullet (Aldrichetta forsteri), and four benthic species, southern longfin goby (Favonigobius lateralis), blue weed whiting (Haletta semifasciata), smooth toad fish (Tetractenos glaber), congolli (Pseudaphritis urvillii), were selected for the study (Supplementary methods). For the behavioural analyses, the two species of mullets were combined to one taxonomic group (see ‘Mesocosm behavioural experiments’ below). The selected seven species are very common across South Australia’s estuaries and coastal zones. Upon collection, fish were acclimated under ambient temperature and pH levels to tank conditions (73 l bins) for 21 days. Subsequently, fish were transferred to outdoor circular mesocosms (1,800 l capacity) where they were kept for 7 days. After the acclimation period, future climate conditions were simulated in a factorial design. A total of 12 mesocosms maintained four treatments (control, ocean acidification, elevated temperature, and the combined ocean acidification and elevated temperature), each with three replicates (Supplementary methods).
Seven individuals from each species were added to each mesocosm, with the exception of hardyheads for which a total of 14 individuals were added per mesocosm. Initially, the hardyheads were considered to represent two species, the small-mouthed hardyhead (Atherinosoma microstoma) and elongated hardyhead (Atherinosoma elongatum). After physiological examination, they were reconsidered as representing small-mouthed hardyheads (Atherinosoma microstoma) as derived from their single developed gonad and tooth patches on the tongue (Ivantsoff and Crowley 1996; Ye et al. 2015).
2.2.2 Mesocosm behavioural experiments
A set of behavioural responses were quantified for each species of fish in response to the experimental treatments after 40 days, i.e. activity levels, bite rate, and boldness. A 50-ml transparent vial with apertures on the sides and covered with mesh was placed in the middle of the mesocosm tank. The vial contained 25 live adult brine shrimps (Artemia salina) as visual cues, and a mixture of food (3 g of blood worms and 1.5 g of blended sardines, shrimp and squid) as olfactory cues. Around midday, fish behaviour was recorded from the top of the tank for 7 min (adopting the approach of Goldenberg et al. (2018) who also showed that 7 min is sufficient to establish behavioural differences under different climate treatments) using a GoPro™ Hero4 Silver camera attached to a PVC frame. Recordings were analysed using VLC (VideoLAN Organization) media player 2.1.3. A frame was overlayed onto the computer screen and divided the field of view in eight areas. The behaviour of individual fish was recorded from the time it entered until it left the field of view. Each time a fish entered the field of view, it was considered a new individual. Activity level was measured as the percentage of time the fish spent swimming. Bite rate was estimated as the number of bites the fish took at the food vial per minute. Boldness was quantified as the percentage time a fish spent in the areas closest (arena area) to the vial. Due to the difficulty off differentiating between gold spot mullet and yellow-eyed mullet in the video analysis, they were categorised into one group as ‘mullets’. Experiments were performed under The University of Adelaide Animal Ethics Committee approval S-2016–087.
2.2.3 Aquarium experimental design
The southern longfin goby and the small-mouthed hardyhead were relocated from the mesocosm to the aquarium room and held in 40-l tanks for an additional 3.2 months. The quality of the seawater was maintained similar to the conditions of the mesocosms, but fish were kept separated by species (Supplementary methods). Due to space restrictions in the laboratory, only two species could be relocated. Behavioural experiments on these two species were also done in aquaria (in addition to those in the mesocosms) to study behavioural responses in small tanks as typically used in climate change experiments on fishes.
2.2.4 Aquarium behavioural experiments
After 3.7 months of treatment exposure (combined mesocosm and aquarium conditions), fish activity levels and bite rates were tested inside the 40-l aquarium tanks. A 50-ml vial with the same characteristics as in the mesocosm experiments was placed in the middle of the tank. The vial contained the same visual (brine shrimps) and olfactory cues (food mixture) described in the mesocosm experiments. Fish behaviour was recorded remotely from the top of the tank for 7 min around midday (similar to the mesocosms), using either a Canon Legria HF-R406 or a Canon Legria HFM52 camera attached to a metal frame. Behaviour was then analysed from the videos using VLC media player 2.1.3 with a grid of eight squares overlapping the tank arena. Activity levels were evaluated as the number of lines crossed by the fish per minute (Munday et al. 2013), while bite rate was quantified as the number of bites at the food vial per minute. Boldness was quantified as the percentage time a fish spent in the areas closest to the vial (arena area). Due to some poorly focused videos, we were only able to evaluate 6 min of the recordings for southern longfin gobies and 5 min for hardyheads. Experiments were performed under The University of Adelaide Animal Ethics Committee approval #S-2016–165.
2.3 Physiological proxies
Physiological indicators were tested within both natural and laboratory systems (aquarium fish only). Because biomarkers, RNA/DNA ratios, and behaviour respond almost immediately to treatment effects, and because fish spent 3.2 months in the aquarium before tissue sampling, these measurements relate to the effects of the aquarium treatment conditions rather than those of the mesocosm.
Stress responses and condition of the fishes were evaluated by assessing different indicators: total antioxidant capacity (TAC), lipid peroxidation or oxidative damage (MDA), RNA/DNA ratio, gonadosomatic index (GSI), hepatosomatic index (HSI), relative condition factor, and somatic growth.
Fish muscle tissue (~ 25 mg for laboratory, ~ 4 mg for vents, and ~ 4.8 mg for natural warming natural systems) was used for the RNA/DNA ratio analyses. The D7001 ZR-Duet™ DNA/RNA MiniPrep Kit (Zymo Research) was used for DNA and RNA extraction. RNA samples were treated with the E1010 DNase I Set (250 U) w/ DNA Digestion Buffer to avoid contamination from DNA into RNA samples. A Quantus Fluorometer was used to quantify the concentration of DNA and RNA from the fish muscle samples. To adjust the quantified value to the weight of the sample, we obtained the total weight of DNA or RNA sample and divided this by the weight of the tissue sample:
Fish muscle tissue (~ 100 mg for laboratory, ~ 15 mg for vents and natural warming systems) was also used to prepare a 10% tissue homogenate in an ice bath, and subsequently used to assess total antioxidant capacity (TAC) and malondialdehyde concentration (MDA, indicative of oxidative damage). Coomassie blue staining method was used to quantify the protein concentration in the 10% tissue homogenate. Assay kits purchased from Nanjing Jiancheng Bioengineering Institute, China, were used to evaluate TAC (CAT no: A015-1) and MDA concentration (CAT no: A003-1), following the manufacturer’s manuals.
The energy reserves of aquarium fishes were calculated based on the hepatosomatic index (HSI). The HSI was calculated based on the wet weight of the liver and of the entire fish:
Liver wet weight was used to estimate the reproductive investment of fishes from the natural systems.
Body condition was calculated for each fish individually using the relative condition factor Kn (Le Cren 1951):
where w is the observed body weight and W is the theoretically estimated weight:
where L is the body length, a is the intercept, and b is the slope. The values of a and b were estimated from log transformed values of weight and length.
For the vent systems, condition could only be estimated for the common triplefin (samples from 2017). Mesocosm and aquarium fish condition was tested at the end of each experiment.
2.4 Statistical analyses
We constructed frequency-distribution plots for all the behavioural and physiological responses in order to visualise the distribution of phenotypes across controls vs CO2 or temperature treatments, respectively. We used a two-sample Kolmogorov–Smirnov test, using the KS-test function in R-Studio v.3.6.0, to test if the control data came from population distributions of the same shape as those from the climate treatments.
Linear models (LM) and generalised linear mixed models (GLMM) were used to test if additional variables affected the behavioural and physiological traits. One model was performed for each behavioural and physiological trait for the natural CO2 vents, for the natural warming sites, and for both mesocosms and aquaria. Assumptions were tested with fitted residual and normality plots. Where assumptions were not met we used a Gamma distribution. We tested the importance score of each variable using a multimodel inference, with a variable importance threshold of 80% (Calcagno and de Mazancourt 2010). Likelihood ratio tests and Chi-square test were used for GLMM’s and LM’s, respectively, to evaluate differences among treatments, species, and sites (only for natural CO2 vents and natural warming), and the interaction between treatments and species.
To test for species variability between control and natural sites or laboratory treatments, we calculated the standard deviation (SD) for boldness of each tested species for both the control and climate treatments. Subsequently, for each system, the mean SD of boldness across species was compared between control and treatments using a t-test (CO2 vents, and natural warming locations) and an ANOVA test (aquarium, mesocosm) using the t.test and aov function respectively in R-Studio v.3.6.0. This test was only performed for boldness as this was the only behaviour that was affected across the majority of species. Additionally, the mean, median, and standard deviation of boldness were compared for each species between controls and climate treatments. A t-test was used to compare the means of CO2 vents and natural warming regions, while the F-test was used to compare their standard deviations. For the laboratory conditions, the means were compared using an ANOVA and Levene’s test for standard deviations. Furthermore, fish densities were measured for each focal species at the natural systems (CO2 vents and warming systems) by visually counting the number of individuals per unit area within belt transects (Nagelkerken et al. 2017; Ferreira et al. 2018).
We calculated the ratio of density change by dividing the density of fishes at naturally elevated CO2 or elevated temperature by their respective density at controls. Similarly, we estimated the ratio of change in boldness (i.e. mean startle distance at CO2 vents or warming systems divided by that at the controls). We tested the relationship between the change in fish density and change in startle distance using least squares linear regression and calculated the R2 of the fitted regression line in R-Studio v.3.6.0. We tested for outliers using Cook’s distance (Cook and Weisberg 1984).
3 Results
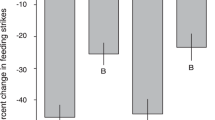
Elevated CO2 drove an increase in frequency of occurrence of bolder individuals relative to present-day conditions. This pattern was consistent for natural CO2 vents (four out of six species, Figs. 1A-E, S2A–B, Table S7) and laboratory aquarium conditions (one out of two species, Fig. 1F, S2C–F). For all these observations, zero to very few shyer individuals remained under elevated CO2 conditions. This pattern was not observed for species from mesocosm systems, where boldness distribution was either similar between elevated CO2 and control conditions (five out of six species, Figs. 1H–I, S2D–F, Table S7), or was reduced under elevated CO2 (one species, Fig. 1G, Table S7). The GLMM test showed that for natural vents, CO2 treatment was a significant explanatory variable for boldness, as well as its interaction with species (Fig. S10a, Table S8).
Boldness frequency distributions of fish from natural CO2 vent systems (A–E), aquaria (F), mesocosms (G–I), and natural warming hotspots (J–K). The x-axis shows a gradient in boldness with shyer individuals positioned towards the left-hand side and bolder individuals toward the right-hand side of each graph, respectively. For natural CO2 vents (A–E) and natural warming hotspots (J–K), boldness was measured as flight initiation distance (cm) towards an approaching artificial threat (see supplementary methods). For aquarium and mesocosms (F–I), boldness was measured as percentage of time spent (sec.) close to an experimental food source. Only graphs showing significant differences among distributions are presented; see Supplementary material for all other graphs. Coloured areas indicate loss or gain of phenotypes. Grey shade: control and cold (at natural warming sites); diamond pattern: elevated CO2; diagonal lines: elevated temperature; area with squares: combined elevated CO2 and temperature. C: control, OA: ocean acidification, T: elevated temperature, OAT: combined elevated CO2 and elevated temperature. Natural warming, C: cold, W: warming. n—number of individuals; p—p-value; ∆—difference between control (or colder seawaters in natural systems) and the climate stressor (temperature or pCO2). See Table S6 for full scientific and common species names
Fish exposed to warmer environments in natural and laboratory systems presented three main responses in the distribution of their boldness phenotypes. First, in cases where their distributions shifted towards an increased frequency of occurrence of bolder individuals combined with a reduced frequency of shyer phenotypes (one out of five species at natural warming systems; Figs. 1J, S2G–I, and one out of two species in aquarium systems; Figs. 1F, S2C, Table S7). Second, in cases where the range of responses in boldness was narrowed, we observed a loss of both shyer and bolder individuals (one out of five species at natural warming systems; Fig. 1K), and consequently a peak of phenotypes with medium boldness values. Third, where there was an increase in the frequency of occurrence of shyer individuals and a reduced frequency of bolder individuals (two out of six species in mesocosms, Fig. 1H, I, Table S7). Fish exposed to the combination of elevated CO2 and warming in aquaria and mesocosms generally showed a distribution similar to that of the controls (Figs. 1G–I, S2C–F, Table S7), or were positioned in between that of elevated temperature and elevated CO2 in isolation (Fig. 1F). The linear model tests showed that for natural warming systems, site was the best explanatory variable for boldness behaviour (Table S10).
For natural systems, within-species phenotypic variance for boldness (Table S6) was lower at CO2 vents (p = 0.049, Fig. 2A) and natural warming hotspots (p = 0.024, Fig. 2B) compared to controls (ambient CO2 and colder water temperature, respectively), but this reduction was not observed under any of the laboratory conditions (mesocosm or aquarium, Fig. 2C, D).
Change in mean variability (1 SD) of boldness phenotypes within species at controls and treatments for (A) natural CO2 vents, (B) natural warming hotspots, (C) mesocosms, and (D) aquaria. C: control; OA: ocean acidification, T: elevated temperature; OAT: combination of ocean acidification and temperature. Error bars represent standard deviation (across species)
There was a significant linear relationship between boldness at CO2 vent sites and the density of the fish (R2 = 0.866, p = 0.007, Fig. 3A; Table S5) when a detected outlier was removed from the analysis (see Table S4 for results with complete data set). For natural warming sites, increased boldness resulted in increased densities for 3 out of 5 species, but no significant linear relationship was found (Tables S4, S5). The frequency distribution of phenotypes of other behavioural traits (activity levels and feeding rate, Figs. S3, S4, Table S7) and of various physiological proxies (body relative condition factor, total cellular antioxidant capacity, cellular oxidative damage, RNA/DNA tissue ratios, and liver weight or hepatosomatic index; Figs. S5–S9, Table S7) generally did not differ between control and treatment conditions (temperature or elevated CO2 or their combination), either in natural or laboratory systems. There were a few exceptions to this observation, but these did not present any consistent patterns (Figs. S3–5, Table S7).
Increases in fish densities in the wild as a function of increased fish boldness for natural analogues of climate stressors. (A) volcanic CO2 vents (one outlier removed); (B) natural warming hotspots. Each dot represents a different species. Fitted regression lines with associated R2-values and p-values are shown (see also Tables S4, S5 for statistical outputs)
4 Discussion
We reveal an increase in risk-taking phenotypes (bolder individuals) among fishes exposed to ocean acidification and warming, with little change in seven other behavioural and physiological phenotypic traits. At least five out of twelve fish species experienced a shift in their trait distribution towards bolder phenotypes when exposed to elevated CO2, with a consequent loss of shyer individuals. When faced with elevated temperature, however, species showed a dual response comprising losses (Atypichthys strigatus, Tetractenos glaber, Atherinosoma microstoma in mesocosm) as well as gains (Acanthurus nigrofuscus, Atherinosoma microstoma in aquarium) of bold phenotypes. Likewise, laboratory studies based on short-term exposures have shown increases (Munday et al. 2010; Biro et al. 2010) and decreases (Hamilton et al. 2013; Rossi et al. 2015) in boldness under ocean acidification and ocean warming. In contrast, the frequency distribution of phenotypic traits related to feeding and physiology was similar under future climate and control conditions across all study systems, with the exception of a few species. In the wild, there was furthermore a reduction in population-level phenotypic variance for boldness in naturally acidified and warming environments. These results suggest that environmental filtering of phenotypes occurs under ocean acidification and warming, but is likely more readily observed in wild populations that are exposed to climate stressors for the majority of their life.
Populations with a greater proportion of bolder individuals occurred in localities of natural ocean warming and acidification. Rather than suffer poorer body condition, individuals within these populations were more densely packed within natural CO2 or warmed systems. Bolder individuals are often more active, dominant, and successful in acquiring food and other resources than their shyer counterparts (Ariyomo and Watt 2012). When competing for resources in a changing environment, populations with bolder individuals could outcompete and outgrow other populations in which risk-taking behaviour is not altered (Kua et al. 2020). Outcompeting shyer individuals for food resources could have allowed bolder organisms to maintain their fitness (Kua et al. 2020) at the ocean warming and acidification sites. Bolder individuals often show positive somatic growth, although their risk of predation increases at the same time (Smith and Blumstein 2008). The natural CO2 vent sites used in this study had reduced densities of predators compared to control sites (Nagelkerken et al. 2016, 2017), providing a potential survival advantage to bolder fishes under elevated CO2. The scarcity of predators at CO2 vents and the increase in food resources could have aided individuals in maintaining their physiological homeostasis (Thomsen et al. 2013; Ramajo et al. 2016; Gobler et al. 2018). In environments with high quantities of food, mobile organisms require less time searching for food resources (Biro et al. 2003), and this allows them to maintain their energy reserves. Likewise, high food availability can buffer against oxidative damage at cellular levels, by providing supplemental energy to enhance the body’s antioxidant capacity and prevent oxidative stress (Rodriguez-Dominguez et al. 2019). Hence, under elevated CO2, an increase in bolder phenotypes could confer the species with greater growth or reproductive success (Nagelkerken et al. 2021), and a competitive advantage for resources over species that do not show such shifts in boldness, ultimately increasing the population size of species that show positive phenotypic adjustments to ocean acidification.
Wild populations under present-day conditions had greater variability in boldness phenotypes compared to those subjected to elevated CO2 or elevated temperature. This was not observed in laboratory systems, possibly due to a difference in species studied, but more likely due to shorter treatment exposure times and/or a smaller selection of possible phenotypes that could fit within the space-limited laboratory tanks. Contrary to our results, O’Dea et al. (2019) reported an increase in phenotypic variability in fishes at higher temperatures in their meta-analysis, although they only analysed studies performed under laboratory conditions. Possibly, this was caused by an increased frequency of rare phenotypes under warming in aquarium experiments (O’Dea et al. 2019), with many of such phenotypes unlikely to persist in the wild. In nature, species interactions and environmental factors can pose selective pressure on phenotypic traits (Sobral et al. 2013). When facing environmental change, the degree of phenotypic variability affects the viability of a population, as a wider range of available phenotypes are more likely to hold a particular plastic response needed in novel or changing environments (Brown et al. 2007; Ariyomo and Watt 2012). As a result, the narrowing of phenotypic variability in the wild can negatively influence populations across evolutionary through ecological scales (Ariyomo and Watt 2012), by reducing the breath of adaptive phenotypes that are suited to future environments altered by additional natural or anthropogenic disturbances. As such, populations where a phenotypic trait is favoured in the environment will face greater risk of decline if natural conditions change, either by climate-related or human stressors.
Risk-taking behaviour was the only trait that was consistently altered in its frequency distribution across climate change stressors and across a variety of species. At naturally elevated CO2 vents, risk-taking phenotypes were distributed toward bolder behaviours. Over generations, if a trait in a population is favoured towards one end of the phenotypic distribution, directional selection can occur (Breed and Moore 2012), resulting in a decrease in population variance, and a change in the mean value of the trait (Kingsolver and Pfenning 2007, Sanjack et al 2018). Selection of a phenotype will only lead to evolutionary changes if the trait is heritable (Kingsolver and Pfenning 2007). Boldness is known to be a highly heritable trait (Ferrari et al. 2016), and therefore environments where climatic stressors are continuously facilitating bolder phenotypes might experience positive selection towards this trait when individual fitness is enhanced. Phenotypic variability in a population allows for the initial selection of particular traits that provide an advantage within the new conditions, but when these advantageous traits are consistently selected in the new environment, they become increasingly dominant in the population, thereby reducing the overall phenotypic variability.
Modification of fish behaviour in a changing environment can have direct or indirect effects on demographic parameters, and in turn alter population dynamics (Wong and Candolin 2015). Changes towards bolder behaviour could allow species to access more resources than their shyer competitors and in turn modify species interactions. Selection towards a larger trait value or a change in its frequency distribution can also modify the patterns of species interactions and natural selection (Bolnick et al. 2011), irrespective of its heritability. Thus, due to the different effects that climate change exerts on fish species and the narrowing of risk-taking phenotypic variation, the strength of interactions in the community can be altered in a future climate scenario. Differences in behavioural responses across species can change the strength and nature of their interactions, such as predation and competition (Wong and Candolin 2015), given that behavioural responses of one species can be linked to the ecological and selective environment of other species (Wolf and Weissing 2012). Consequently, differential shifts in the distribution of bolder phenotypes across species under changing environments could have an indirect impact on the structure of species communities, through reductions of less dominant species.
Understanding how phenotypic plasticity alters species adjustments to climate change is key to recognising their capacity to acclimate and persist under future environments. We demonstrate that global change can modify and narrow the distribution of bolder phenotypes in fishes, particularly under ocean acidification. Future changes in climate can put populations under selective pressure and modify the community structure of species. Consequently, altered distributions of shyer and bolder behavioural phenotypes can modify the interaction between species, strengthening the dominance of some species over others, and opening a pathway towards more homogenised communities.
Data availability
Data supporting the analyses will be made available as Supplementary material upon acceptance.
References
Ariyomo TO, Watt PJ (2012) The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim Behav 83:41–46. https://doi.org/10.1016/j.anbehav.2011.10.004
Assis APA, Patton JL, Hubbe A, Marroig G (2016) Directional selection effects on patterns of phenotypic (co)variation in wild populations. Proc R Soc B 283:20161615. https://doi.org/10.1098/rspb.2016.1615
Barker D, Allan GL, Rowland SJ, Pickles JM (2002) A guide to acceptable procedures and practices for aquaculture and fisheries research, 2nd Edition. Prepare for the NSW Primary Industries (fisheries) Animal Care and Ethics Committee, Industry and Investment NSW, Port Stephens
Bellard C, Bertelsmeier C, Leadly P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15(4):365–377. https://doi.org/10.1111/j.1461-0248.2011.01736.x
Biro PA, Beckmann C, Stamps JA (2010) Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc Biol Sci 277(1678):71–77. https://doi.org/10.1098/rspb.2009.1346
Biro PA, Post JR, Parkinson EA (2003) From individuals to populations: prey fish risk-taking mediates mortality in whole-system experiments. Ecology 84:2419–2431
Bolnick DI, Amarasekare P, Araujo MS, Burger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur DA (2011) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26:183–192
Bonamour S, Chevin LM, Charmantier A, Teplitsky C (2019) Phenotypic plasticity in response to climate change: the importance of cue variation. Phil Trans r Soc b 374:20180178. https://doi.org/10.1098/rstb.2018.0178
Booth DJ, Figueira WF, Gregson MA, Brown L, Beretta GA (2007) Occurrence of tropical fishes in temperate southeastern Australia: role of the East Australian Current. Estuar Coast Shelf Sci 72:102–114
Booth DJ, Beretta GA, Brown L, Figueira WF (2018) Predicting success of range expanding coral reef fish in temperate habitats using temperature-abundance relationships. Front Mar Sci 5(31):1–7. https://doi.org/10.3389/fmars.2018.00031
Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP, Gehlen M, Vichi M (2013) Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10:6225–6245
Breed M, Moore J. Mating systems. 2012. In: Animal Behaviour. Second edition. https://doi.org/10.1016/B978-0-12-801532-2.00011-8
Brown C, Burgess F, Braithwaite VA (2007) Heritable and experiential effects on boldness in a tropical poeciliid. Behav Ecol Sociobiol 62:237–243. https://doi.org/10.1007/s00265-007-0458-3
Buskirk JV (2017) Spatially heterogeneous selection in nature favours phenotypic plasticity in anuran larvae. Evolution 71(6):1670–1685
Calcagno V, de Mazancourt C (2010) glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Software 34(12):1–29. https://doi.org/10.18637/jss.v034.i12
Caldeira K, Wickett M (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425:365–365
Connell SD, Doubleday ZA, Foster NR, Hamlyn SB, Harley CDG, Helmuth B, Kelaher BP, Nagelkerken I, Rodgers KL, Sarà G, Russell BD (2018) The duality of ocean acidification as a resource and a stressor. Ecology 99:1005–1010
Cook RD, Weisberg S (1984) Residuals and influence in regression. Wiley. Fox, J. 1997. Applied Regression, Linear Models, and Related Methods. Sage. Williams, D. A. (1987) Generalized linear model diagnostics using the deviance and single case deletions. Appl Stat 36:181–191
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res Part A, Oceanogr Res Pap 34(10):1733–1743
Domenici P, Blake RW (1997) The kinematics and performance of fish fast-start swimming. J Exp Biol 200:1165–1178
Donelson JM et al (2019) Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Phil Trans r Soc b 374:20180186
Edelaar P, Jovani R, Gomez-Mestre I (2017) Should I change or should I go? Phenotypic plasticity and matching habitat choice in the adaptation to environmental heterogeneity. Am Nat 190(4):506–520
Ferrari S, Horri K, Allal F, Vergnet A, Benhaim D, Vandeputte M et al (2016) Heritability of boldness and hypoxia avoidance in European Seabass. Dicentrarchus Labrax Plos ONE 11(12):e0168506. https://doi.org/10.1371/journal.pone.0168506
Ferreira CM, Nagelkerken I, Goldenberg SU, Connell SD (2018) CO2 emissions boost the benefits of crop production by farming damselfish. Nat Ecol Evol 2:1223–1226
Figueira WF, Biro P, Booth DJ, Valenzuela VC (2009) Performance of tropical fish recruiting to temperate habitats: role of ambient temperature and implications of climate change. Mar Ecol Prog Ser 384:231–239. https://doi.org/10.3354/meps08057
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21(3):394–407
Gibert JP, Brassil DE (2014) Individual phenotypic variation reduces interactions strengths in a consumer-resource system. Ecol Evol 4(18):3703–3713
Gobler CJ, Merlo LR, Morrell BK, Griffith AW (2018) Temperature, acidification, and food supply interact to negatively affect the growth and survival of the forage fish, Meniia beryllina (inland silverside), and Cyprinodon variegatus (sheepshead minnow). Front Mar Sci 5:86. https://doi.org/10.3389/fmars.2018.00086
Goldenberg SU, Nagelkerken I, Marangon E, Bonnet A, Ferreira CM, Connell SD (2018) Ecological complexity buffers the impacts of future climate on marine consumers. Nat Clim Chang 8:229–233
Hamilton TJ et al (2013) CO2-induced ocean acidification increases anxiety in Rockfish via alteration of gamma-amminobutyric acid type A receptor functioning. Proc R Soc B 281(1775):20132509
Henn JJ, Buzzard V, Enquist BJ, Halbritter AH, Klanderud K, Maitner BS, Michaletz ST, Pötsch C, Seltzer L, Telford RJ, Yang Y, Zhang Land Vandvik V (2018) Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 9, 1548
Herczeg G, Gonda A, Merila J (2009) Predation mediated population divergence in complex behaviour of nine-spined stickleback (Pungitius pungitius). J Evol Biol 22:544–552
Intergovernmental Panel on Climate Change (IPCC) (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J (eds) Contribution of working group I to the Fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, p 1535. http://www.climatechange2013.org/
Ivantsoff W, Crowley LELM (1996) Family Atherinidae, silversides or hardyheads. In: McDowall RM (ed) Freshwater fishes of South-eastern Australia, 2nd edn. Reed Books, Sydney, pp 123–133
Kingsolver JG, Pfennig DW (2007) Patterns and power of phenotypic selection in nature. Bioscience 57:561–572
Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM (2015) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–599. https://doi.org/10.1111/1365-2435.12345
Kua ZX, Hamilton IM, McLaughlin AL et al (2020) Water warming increases aggression in a tropical fish. Sci Rep 10:20107. https://doi.org/10.1038/s41598-020-76780-1
Lafuente E, Beldade P (2019) Genomics of developmental plasticity in animals front. Genet 10:720
Laubenstein T, Rummer J, Nicol S, Parsons D, Pether S, Pope S, Smith N, Munday P (2018) Correlated effects of ocean acidification and warming on behavioral and metabolic traits of a large pelagic fish. Diversity 10(2):35. https://doi.org/10.3390/d10020035
Le Cren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol 20(2):201–219
Leung JYS, Russell BD, Connell SD (2019) Adaptive responses of marine gastropods to heatwaves. One Earth 1:374–381
Lozada-Gobilard S, Stang S, Pirhofer-Walzl K, Kalettka T, Heinken T, Scröder B, Eccard J, Jpshi J (2019) Environmental filtering predicts plant-community trait distribution and diversity: kettle holes as models of meta-community systems. Ecol Evol 9:1898–1910. https://doi.org/10.1002/ece3.4883
Matesanz S, Gianoli E, Valladares F (2010) Global change and the evolution of phenotypic plasticity in plants. Ann N Y Acad Sci 1206:35–55. https://doi.org/10.1111/j.17496632.2010.05704.x
Mehrbach C, Culberson CH, Hawley JE, Pytkowicx RM (1973) Measurement of apparentdissociation-constants of carbonic-acid in seawater at atmospheric-pressure. Limnol Oceanogr 18:897–907. https://doi.org/10.4319/lo.1973.18.6.0897
Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP, Karl D (2010) Replenishment of fish populations is threatened by ocean acidification. Proceed National Acad Sci USA 2010(107):12930–12934
Munday PL, Pratchett MS, Dixson DL, Donelson JM, Endo GGK, Reynolds AD, Knuckey R (2013) Elevated CO2 affects the behavior of an ecologically and economicallyimportant coral reef fish. Mar Biol 160(8):2137–2144. https://doi.org/10.1007/s00227-012-2111-6
Nagelkerken I, Connell SD (2015) Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc Natl Acad Sci U S A 112(13):272–13277
Nagelkerken I, Munday P (2016) Animal behaviour shapes the ecological effects of ocean acidification and warming moving from individual to community-level responses. Glob Chang Biol 22:974–989. https://doi.org/10.1111/gcb.13167
Nagelkerken I, Russell BD, Gillanders BM, Connell SD (2016) Ocean acidification alters fish populations through habitat modification. Nat Clim Chang 6:89–93. https://doi.org/10.1038/nclimate2757
Nagelkerken I, Goldenberg SU, Ferreira CM, Russell BD, Connell SD (2017) Species interactions drive fish biodiversity loss in a high-CO2 world. Curr Biol 27:2177–2184. https://doi.org/10.1016/j.cub.2017.06.023
Nagelkerken I, Goldenberg SU, Coni E, Connell SD (2018) Microhabitat change alters abundances of competing species and decreases species richness under ocean acidification. Sci Total Environ 645:615–622. https://doi.org/10.1016/j.scitotenv.2018.07.168
Nagelkerken I, Alemany T, Anquetin JM, Ferreira CM, Ludwig KE, Sasaki M, Connell SD (2021) Ocean acidification boosts reproduction in fish via indirect effects. PLoS Biology 19:e3001033
Nunney L (2016) Adapting to a changing environment: Modelling the interaction of directional selection and plasticity. J Hered 107(1):15–24
O’Dea R, Lagisz M, Hendry A, Nakawa S (2019) Developmental temperature affects phenotypic means and variability: a meta-analysis of fish data. Fish and Fisheries 20(5):1005–1022. https://doi.org/10.1111/faf.12394
Pierrot D, Lewis E, Wallace DWR (2006) MS excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center. Oak Ridge National Laboratory; US Department of Energy, Oak Ridge, Tennessee. https://doi.org/10.3334/CDIAC/otg.CO2SYS_XLS_CDIAC105a
Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20:481–486
Poloczanska ES, Babcock RC, Butler A, Hobday AJ, Hoegh-Guldberg O, Kunz TJ, Matear R, Milton D, Okey TA, Richardson AJ (2007) Climate change and Australian marine life. Oceanogr Mar Biol Ann Rev 45:407–478
Ramajo L, Pérez-León E, Hendriks I et al (2016) Food supply confers calcifiers resistance to ocean acidification. Sci Rep 6:19374. https://doi.org/10.1038/srep19374
Reed TE, Schindler DE, Waples RS (2011) Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conserv Biol 25(1):56–63
Rodriguez-Dominguez A, Connell SD, Leung YS, Nagelkerken I (2019) Adaptive responses of fishes to climate change: feedback between physiology and behaviour. Sci Total Environ 692:1242–1249. https://doi.org/10.1016/j.scitotenv.2019.07.226
Rossi T, Nagelkerken I, Simpson SD, Pistevos JCA, Watson S-A, Merillet L, Fraser P, Munday PL, Connell SD (2015) Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc R Soc B 282:20151954. https://doi.org/10.1098/rspb.2015.1954
Sanjack JS, Sidorenko J, Robinson M, Thornton K, Visscher P (2018) Evidence of directional and stabilizing selection in contemporary humans. PNAS 115(1):151–156
Scheiner SM (1993) Genetics and evolution of phenotypic plasticity. Annu Rev Ecol, Evol, Syst 24:35–68
Schmid M, Guillaume F (2017) The role of phenotypic plasticity on population differentiation. Heredity 119:214–225
Schunter C, Welch MJ, Ryu T, Zhang H, Berumen ML et al (2016) Nat Clim Chang 6:1014–1018. https://doi.org/10.1038/nclimate3087
Smith BR, Blumstein DT (2008) Fitness consequences of personality: a meta-analysis. Behav Ecol 19(2):448–455. https://doi.org/10.1093/beheco/arm144
Sobral M, Guitian J, Guitian P, Larrinaga AR (2013) Selective Pressure along a Latitudinal Gradient Affects Subindividual Variation in Plants. PLos One 8(9):e74356. https://doi.org/10.1371/journal.pone.0074356
Souza ML, Duarte AA, Lovato MB, Fagundes M, Valladares F, Lemos-Filho JP (2018) Climatic factors shaping intraspecific leaf trait variation of a neotropical tree along a rainfall gradient. PLoS ONE 13(12):e0208512. https://doi.org/10.1371/journal.pone.0208512
Sultan SE, Spencer HG (2002) Metapopulation structure favors plasticity over local adaptation. Am Nat 160(2):271–83. https://doi.org/10.1086/341015
Syms C, Jones GP (1999) Scale of disturbance and the structure of a temperate fish guild. Ecology 80(3):921–940
Thomsen J, Casties I, Pansch C, Kortzinger A, Melzner F (2013) Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob Chang Biol 19:1017–1027. https://doi.org/10.1111/gcb.12109
Valladares F, Gianoli E, Gomez JM (2007) Ecological limits to plant phenotypic plasticity. New Phytol 176(4):749–63. https://doi.org/10.1111/j.1469-8137.2007.02275.x
Valladares F et al (2014) The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett 17:1351–1364
White JR, Meekan MG, McCormick MI, Ferrari MCO (2013) A comparison of measures of boldness and their relationships to survival in young fish. PLoS ONE 8(7):e68900. https://doi.org/10.1371/journal.pone.0068900
Wittmann AC, Pörtner HO (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Chang 3:995–1001
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461
Wong BBM, Candolin U (2015) Behavioral responses to changing environments. Behav Ecol 26:665e673. https://doi.org/10.1093/beheco/aru183
Ye Q, Bucater L, Short D (2015) Coorong fish condition monitoring 2008–2014: black bream (Acanthopagrus butcheri), greenback flounder (Rhombosolea tapirina) and smallmouthed hardyhead (Atherinosoma microstoma) populations. South Australia Research and Development Institute (Aquatic Sciences), Adelaide (SARDI Publication No. F2011/000471–4. SARDI Research Report Series No. 836. 1005)
Acknowledgements
We thank all the students and interns for their assistance during field trips and maintenance of the mesocosms and aquarium tanks, and behavioural experiments in particular Renske Jongen, Jasper Bunschoten, Julie Anquetin, Tiphaine Alemany, Kim Ludwig, Camilo Ferreira, Kelsey Kingsbury, Clement Baziret, and Judith Giraldo.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This study was financially supported by an Australian Research Council (ARC) Future Fellowship (grant no. FT120100183) and a grant from the Environment Institute (The University of Adelaide) to I.N., an ARC Discovery Project (grant no. DP170101722) to I.N. and D.J.B., and an ARC Discovery Project (grant no. DP150104263) to S.D.C. A.R.D. was sponsored by a CONACYT (the National Council of Science and Technology, no. 409849) scholarship from Mexico.
Author information
Authors and Affiliations
Contributions
IN and SDC designed the natural CO2 seep experiments. IN, DB, EC, and MS designed the natural warming hotspots experiments. ARD and IN designed the mesocosm and aquarium experiments. ARD and MS performed some of the laboratory analyses. ARD analysed the data and performed the statistical analyses. ARD, SC, and IN wrote the manuscript. DB, EC, and MS contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Consent to participate
Informed consent was obtained from all authors to participate.
Consent for publication
Informed consent was obtained from all authors to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodriguez-Dominguez, A., Connell, S.D., Coni, E.O.C. et al. Phenotypic responses in fish behaviour narrow as climate ramps up. Climatic Change 171, 19 (2022). https://doi.org/10.1007/s10584-022-03341-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10584-022-03341-y