Abstract
As the global climate warms, the fate of lacustrine fish is of huge concern, especially given their sensitivity as ectotherms to changes in water temperature. The Arctic charr (Salvelinus alpinus L.) is a salmonid with a Holarctic distribution, with peripheral populations persisting at temperate latitudes, where it is found only in sufficiently cold, deep lakes. Thus, warmer temperatures in these habitats particularly during early life stages could have catastrophic consequences on population dynamics. Here, we combined lake temperature observations, a 1-D hydrodynamic model, and a multi-decadal climate reanalysis to show coherence in warming winter water temperatures in four European charr lakes near the southernmost limit of the species’ distribution. Current maximum and mean winter temperatures are on average ~ 1 °C warmer compared to early the 1980s, and temperatures of 8.5 °C, adverse for high charr egg survival, have frequently been exceeded in recent winters. Simulations of winter lake temperatures toward century-end showed that these warming trends will continue, with further increases of 3–4 °C projected. An additional 324 total accumulated degree-days during winter is projected on average across lakes, which could impair egg quality and viability. We suggest that the perpetuating winter warming trends shown here will imperil the future status of these lakes as charr refugia and generally do not augur well for the fate of coldwater-adapted lake fish in a warming climate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Owing to their intrinsic relationship with the overlying atmosphere and surrounding landscape, lakes are one of the most sensitive habitats to changes in climate. Mounting evidence has revealed that over the past century, freshwater lake temperatures have risen, owing to the secular trend in global climate (Livingstone 2003; O’Reilly et al. 2015; Woolway et al. 2019). Some biogeochemical and biological consequences of warming lake temperatures have already been documented, including oxygen depletion at depth (e.g. Jankowski et al. 2006), decreased carbon sequestration (e.g. Yvon-Durocher et al. 2017), and changing phyto- (e.g. Reavie et al. 2017) and zooplankton (e.g. Carter et al. 2017) population dynamics. Alarmingly, this widespread trend of warming lakes is anticipated to continue unabated (Shatwell et al. 2019; Woolway and Merchant 2019), and the associated ecological consequences of future temperature regimes require urgent evaluation.
Of particular concern is the fate of lacustrine fish, ectothermic organisms that are highly sensitive to ambient temperatures and have limited potential to shift their geographical distributions. From a societal and ecological perspective, the coldwater salmonids (the trout, salmon, coregonid, and charr species) are the emblematic freshwater fish of the Northern Hemisphere. Within lake ecosystems, salmonids fulfil several important trophic roles, both as predator and prey, and anadromous morphs can transport nutrients from marine areas to lakes, rivers, and headwaters (e.g. Joy et al. 2020). Salmonids are often culturally symbolic and economically important for tourism and recreation (e.g. Butler et al. 2009; Winfield et al. 2019). Thus, documenting the potential negative consequences of climate change on these fish can serve as a powerful tool to motivate public concern and action toward mitigating climate change impacts.
The Arctic charr (Salvelinus alpinus L.) is a cold stenothermic salmonid and the world’s northernmost freshwater fish, with a Holarctic distribution (Whiteley et al. 2019). Lake-resident peripheral charr populations exist at temperate latitudes, albeit with a much reduced distribution compared to Arctic populations. Owing to their thermal requirements, they are generally found only in suitably cold and deep lakes situated in Central Europe and the North Atlantic Isles and are considered a ‘relict’ species of the last ice age (Whiteley et al. 2019). However, southern lake-resident charr populations have undergone significant declines which has been attributed to numerous co-existing stressors, including eutrophication and introduction of non-native fish species (e.g. Maitland et al. 2007; Winfield et al. 2008). Long-term climate warming has been identified as a direct threat to the remaining charr populations of British (Winfield et al. 2010) and peri-alpine lakes (Gerdeaux 2011). Although suggested not to be a direct cause for charr declines in Irish lakes, climate warming may increase the vulnerability of charr populations in shallow lakes with non-salmonid fish communities (Connor et al. 2019).
In aquatic ectotherms, the spawning adult and embryonic egg stages have the narrowest thermal tolerance range and are therefore most vulnerable to warming temperatures (Elliott and Elliott 2010; Dahlke et al. 2020). Warm winters can subject fertilised embryos to adverse conditions, leading to smaller egg sizes, lower hatch success, and smaller emergent larvae (Farmer et al. 2015). In the southern peripheral Arctic charr lakes of Europe, spawning typically occurs in late autumn and winter and requires stone and gravel substrate, which is frequently restricted to specific depth zones in each lake (Gillet 1991; Low et al. 2011; Miller et al. 2015; Gerdeaux 2011). The STOREFISH database (Teletchea et al. 2007), a repository of reproductive traits of freshwater teleosts based on published literature, provides data for the upper thermal tolerance of Arctic charr eggs. Multiple studies concur that egg mortality increases abruptly at water temperatures > 8–9 °C, and these studies encompass a range of freshwater populations from different geographic regions across the southern distribution limit of charr including Britain (Swift 1965), Central European alpine lakes (Jungwirth and Winkler 1984; Humpesch 1985; Crisp 1996; Mari et al. 2016), and Canada (Kerr and Grant 2000). Furthermore, eggs that do survive warmer incubation temperatures (8.5 °C) showed poorer development in terms of embryo size relative to those incubated at 5 °C (Mari et al. 2016).
Such sensitivity to temperature change makes peripheral Arctic charr populations an ideal sentinel for assessing climate change impacts on lake ecosystems. Conserving remnant southern charr populations would maintain a unique source of biodiversity in each individual lake. Such biodiversity can help stabilise ecosystem processes (Schindler et al. 2010), and each individual population, with diverse life-history traits and local adaptations, in turn contributes to the overall biocomplexity of the species (Hilborn et al. 2003). However, several studies to date on the effect of global warming on lake charr have used either air temperature (e.g. Connor et al. 2019), geographical location (e.g. Winfield et al. 2010), or mean annual water temperatures derived from fortnightly profiles (e.g. Gerdeaux 2011) as a proxy for lake warming. Warming rates in lakes may differ from warming air temperature rates for the same region (e.g. Schneider and Hook 2010); the profundal zone temperature is dependent upon specific lake physics, especially in deeper lakes; and lake warming rates are not uniform across seasons (e.g. Woolway et al. 2019). Thus, there is a need to quantify specific rates of warming at biologically relevant depth zones and critical temporal periods and to assess the magnitude of future warming in order to make conclusive claims that climate change represents a direct threat to southern latitude Arctic charr populations.
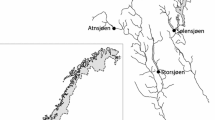
The aim of this study was to characterise changes in the contemporary and future thermal regimes during the winter spawning season of four peripheral European charr populations. These were chosen to represent different Arctic charr lake types at the southernmost distribution of the species’ range—two peri-alpine lakes (Lakes Bourget and Geneva) from Central Europe and two lakes from Ireland and Britain (Lough Bunaveela and Windermere) (Fig. 1). Accurate assessment of temperature dynamics of the depth zones where spawning occurs and not just near the lake surface was crucial. Firstly, we used a hydrodynamic model and gridded climate reanalysis datasets to evaluate whether lake thermal dynamics have already been modified by contemporary climate change over the past four decades and whether temperature increases have encroached upon the thermal tolerance of Arctic charr. Multi-year observations of full water column temperatures from each lake were used to validate fidelity of model simulations to actual in-lake thermal conditions. Secondly, we assessed future changes in the thermal regime of each lake, by forcing each lake model with future atmospheric simulations from four different general circulation models (GCMs) under different emission scenarios. We suggest that results could have importance for evaluating long-term prospects for peripheral Arctic charr populations at southern latitudes in particular and for coldwater lake fish in general in the face of global climate change.
2 Methods
2.1 Study sites
The location and general characteristics of the Arctic charr lakes examined, none of which experiences seasonal ice cover, are shown in Table 1 and Fig. 1. Each lake was selected on the basis of availability of full water column temperature observations for purposes of model performance validation. Lough Bunaveela had full water column temperature observations available at an hourly resolution from 2009 to 2019 and Windermere for 2008 and 2009. The peri-alpine Lake Bourget and Lake Geneva have full water column profiles of temperature available at a bi-monthly or monthly frequency for multiple years (Rimet et al. 2020).
The resident charr populations are considered indigenous to each lake. Following drastic declines in the Arctic charr of Lakes Bourget and Geneva during the twentieth century, presumably due to eutrophication, large-scale restocking of the native populations with allochthonous eggs occurred in the early 1980s (Cachera et al. 2010; Caudron et al. 2014; Savary et al. 2017). A single documented introduction into Bunaveela with an Icelandic strain of charr eggs occurred in 1971 (Piggins and Went 1971), although the population is most likely native—the population has been self-sustaining since at least the 1970s and Bunaveela shares many specific physical characteristics with other native Irish charr lakes (Igoe and Hammar 2004). No restocking of Windermere has been implemented, and the biology and ecology of the native Windermere charr and its subpopulations of spring and autumn spawners have been extensively studied for over half a century (Maitland et al. 2007). We focused on the south basin of Windermere, where the charr have undergone more significant declines relative to the north basin (Winfield et al. 2008). Of Windermere’s spring and autumn spawning subpopulations, autumn spawning individuals are by far the most abundant and dominate the overall lake population (Miller et al. 2015).
2.2 Hydrodynamic model
The lake branch of the open-source General Ocean Turbulence Model (GOTM) was used to simulate water column thermal structure (Burchard et al. 1999). GOTM is a one-dimensional, second-order turbulence closure model. GOTM was set up using the k-ε model scheme, which solves the transport equations for the turbulent kinetic energy (k) and the turbulence dissipation rate (ε), the turbulence length scale determining variable (Burchard and Bolding 2001). Meteorological data used to force GOTM in this study included air temperature (°C), solar radiation (W m−2), wind speed (m s−1) and direction, relative humidity (%), and surface pressure (hPa). Surface heat fluxes were computed using the method of Fairall et al. (1996). GOTM was configured to simulate an enclosed water body with depth–area relations using the hypsograph of each lake as input data.
To assess contemporary lake thermal dynamics, hourly timeseries of meteorological variables were extracted from the 0.25° by 0.25° grid containing each lake from the global ERA5 climate reanalysis dataset (Hersbach et al. 2020) and used as model boundary conditions. In order to maximise model simulation fidelity to observed in-lake conditions, an auto-calibration routine was carried out which allows adjustment of model input parameters in order to minimise error between modelled and observed temperatures. This allows fine-tuning of specific model drivers that may not be fully realistic representations of local meteorological variables at the lake site (e.g. discrepancies between the gridded ERA5 data and actual above-lake meteorological conditions). Model performance was assessed by pairwise comparison between simulated temperatures and measured temperatures at each depth for which observations were available. Calibration for each lake was performed using a subset of annual lake temperature observations, and the calibrated model was validated using a separate set of annual lake temperature observations as test data. Further details of the model calibration and validation procedure are outlined in the Supplementary Material.
2.3 Model simulations: hindcast and forecast
To assess recent historical lake winter temperature trends, each lake model was configured to provide a timeseries of water temperature at 1-m depth intervals from the lake surface down to the maximum depth, at an hourly resolution over the period January 1979 (earliest extent of the ERA5 reanalysis as of August 2020) to December 2019. To assess future temperature dynamics, each lake model was driven using separate sets of meteorological input variables from four different GCM projections: GFDL-ESM2M, HadGEM2-ES, IPSL-CM5A-LR, and MIROC5. Using data from four individual GCMs allows a more robust estimate of future climate compared to using a single GCM, which would increase uncertainty of projections. Each GCM was bias-corrected to the EWEMBI reference dataset (Lange 2019), for the 0.5° grid containing each lake and obtained from the Inter-Sectoral Impact Model Intercomparison Project (ISIMIP2b, Frieler et al. 2017). This bias-correction step overcomes many of the limitations of using global-scale circulation models to simulate meteorological conditions on a local scale (Hempel et al. 2013). Lake model simulations were performed using the following datasets from each of the four GCMs: a historic (1976–2005) reference period and three future climate periods (2020–2099) under different emission scenarios (representative concentration pathway (RCP) 2.6 and RCP 6.0 and RCP 8.5). RCPs relate future concentrations of atmospheric greenhouse gases to radiative forcing in physical models of the future climate system. RCP 2.6 represents a low-end forcing scenario (where mitigation causes a peak and decline in future radiative forcing by century-end), RCP 6.0 a more severe forcing scenario (where diminished mitigation of emissions leads to rising radiative forcing and stabilisation only after century-end), and RCP 8.5 the most aggressive emission scenario in terms of fossil fuel use (leading to continued rising radiative forcing beyond century-end) (Van Vuuren et al. 2011).
2.4 Metrics of lake thermal dynamics relevant to Arctic charr
To assess whether warming trends during the critical egg development stage were apparent over the past four decades, we analysed water temperatures at the known spawning site depths from November to February to cover the period of egg incubation. In Bourget (Cachera et al. 2010) and Geneva (Rubin and Buttiker 1992; Mari et al. 2016), the peak spawning season occurs during December, at depth ranges between 40 and 100 m. In Windermere, both autumn (historical peak in November) and spring (historical peak in February) spawning subpopulations in the south basin appear to spawn at depths shallower than 5 m (Miller et al. 2015). The precise spawning time of Bunaveela charr is unknown; however, charr have been shown to spawn during December in three other Irish lakes at depths < 2 m (Low et al. 2011). Bunaveela charr are likely to spawn at depths shallower than 3 m based on littoral substrate. For each winter period, the mean daily temperature and maximum daily temperature were first calculated from the hourly temperature timeseries from November to February. An overall mean winter temperature and the absolute maximum temperature reached were then calculated from the daily timeseries to give a single mean and max value for each winter in the 40-year timeseries. To assess whether temporal trends could be adequately captured by using a linear regression model, each timeseries was initially fit using a generalised additive model containing a smoothing function of year as a predictor variable, using penalised cubic regression splines, with optimal degree of smoothing estimated using cross-validation (Wood 2017). Model residuals were assessed for normality, heteroscedasticity, and serial auto-correlation. Whether significant trends could be adequately described by using a linear model was ultimately determined by comparing the output of an ordinary least-squares regression and the generalised additive model using analysis of variance and an F test between models. Given the number of studies supporting the increased egg mortality at temperatures exceeding 8–9 °C, the longest consecutive time period in days with water temperatures exceeding 8.5 °C per historical spawning season (November and December) was computed. We used this as a simple indicator of the thermal environment experienced during the early life stage over the past 40 years in each lake. For Lakes Bourget and Geneva, we performed this set of analyses for water temperatures at typical spawning depths of 40 m, 60 m, and 80 m, for Bunaveela at 2 m, and for Windermere at 5 m.
For the future climate simulations, the mean annual winter (November–February) water temperature at the typical spawning depths was calculated for each GCM individually for the historic reference period (1976–2005) and a future climatic period (2020–2099) under each RCP scenario. Future winter temperature anomalies for each lake were determined as the departure in future temperature values from the historic reference period. Anomalies were then averaged across each of the four GCMs to give a mean ensemble representative value under the RCP 2.6, 6.0, and 8.5 emission scenarios. In addition, the accumulated degree-day (ADD) anomaly during each November–February winter period was calculated for each lake using the same method. ADD was first calculated as ADD = mean daily temperature × number of days from November 1st and future anomalies computed based on the departure from average ADD winter values during the 1976–2005 period. ADD was considered to be a more biologically relevant thermal metric (compared to overall mean winter temperature for example), as it provides a simple indicator of how much more heat, over an equal winter time duration, will be accumulated by developing charr embryos under future climatic scenarios.
3 Results
3.1 Winter thermal dynamics 1979–2019
Comparison of lake model temperature simulations driven by ERA5 reanalysis data with observed in situ temperature profiles available for the same time period showed close agreement for all study lakes, with an average r2 = 0.96, RMSE = 0.65 °C, and MAE = 0.45 °C (see supplementary material Table S1 and Fig. S1). A timeseries of water temperature at surface, intermediate, and deep depth zones for each lake shows the performance of model simulations at different depth zones (Fig. 2). Of particular relevance to the current study was the performance of the model at depths associated with charr spawning (Fig. 2—blue for Bourget (a) and Geneva (c) and black for Bunaveela (b) and Windermere (d)), which appeared satisfactory. The high fidelity of lake models when forced with gridded ERA5 climate data was considered sufficient for providing realistic hindcasts of lake thermal dynamics back to 1979 in each lake.
Timeseries comparison between observed (line) and modelled (symbol) lake temperatures. ‘x’ indicates model calibration period, and ‘o’ indicates model validation period, and colour indicates depth range over which modelled and observed temperatures were averaged. a Bourget (black = 1–5 m, red = 6–25 m, blue = 30–80 m). b Bunaveela (black = 1–3 m, red = 15–18 m). c Geneva (black = 1–5 m, red = 6–25 m, blue = 30–80 m). d Windermere (black = 1–5 m, red = 6–20 m, blue = 20–35 m)
Analysis of annual winter lake temperature timeseries across the 40-year hindcast period revealed coherent warming across lakes at depths where charr spawning is known to occur (Fig. 3; Fig. S2; Table 1). Trends in each timeseries were often non-linear (e.g. Lakes Bourget and Geneva), and for ease of comparison across lakes, we fit generalised additive models to quantify each trend. Estimated trend change over the 40-year period was then determined by computing the difference between the beginning and end of the fitted trend line. Maximum simulated winter temperatures showed significant increases for all lakes and were on average 1.1 °C warmer in 2019 compared to the start of each hindcast period (ranging from 0.6 in Lake Geneva to 1.65 °C in Windermere (Fig. 3, Table 1)). Mean simulated winter temperatures also showed similar warming trends, with mean winter temperatures warming by ~ 1 °C over the course of the 40-year period, when averaged across all lakes with significant trends (Fig. S2, Table 1). Mean winter warming trend over 1980–2019 was non-significant in Bunaveela, which is in large part due to the anomalously cold winters of 2009/10 and 2010/11 (e.g. December 2010 was the coldest December on record in Ireland (Met Éireann, unpublished data). For example, a trend estimated for 1980 to 2009 showed a significant mean winter temperature increase of ~ 1 °C).
In Bunaveela and Windermere, no single winter prior to 1995 had more than 30 consecutive days during the winter spawning period with maximum daily simulated water temperatures in exceedance of 8.5 °C (Fig. 4b and d). In contrast, since 1995, Bunaveela has had three winters and Windermere has had five winters with more than 30 or more consecutive days with water temperature exceeding 8.5 °C. At 40-m depth, Lake Geneva had no winter water temperatures exceeding 8.5 °C during the 1980s but has since shown indication that water temperatures in exceedance of 8.5 °C are becoming frequent, particularly in the most recent 6 winters (Fig. 4c). Of note, no water temperatures in exceedance of 8.5 °C were found to occur at 60 m or 80 m. In contrast to the other 3 lakes, no winter temperatures exceeded 8.5 °C at any depth in the range 40–80 m in Lake Bourget (Fig. 4a).
3.2 Future winter temperature and accumulated degree-day anomalies
Projected mean lake winter temperature changes at spawning site depths (averaged over the range 40–80 m for Lakes Bourget and Geneva) showed increased warming relative to the 1976–2005 reference period (Fig. 5). The RCP 2.6 warming scenario showed similar warming anomaly patterns for Lakes Bourget and Geneva, with warming projected to remain within 1–2 °C (Fig. 5a and c). For Lough Bunaveela and Windermere under RCP 2.6, warming appeared to peak around 2060–2070, increasing above a 1 °C warming anomaly, before declining and stabilising toward century-end (Fig. 5b and d). However, under the more severe RCP 8.5 warming scenario, positive temperature anomalies continued to rise beyond 2060 for all four lakes toward century-end with mean winter temperature projected to increase by ~ 3 °C in Bunaveela and Windermere and by up to 4 °C in Lakes Bourget and Geneva by 2100.
Projected timeseries of mean winter temperature anomalies at spawning depths (2 m for Bunaveela, 5 m for Windermere, and averaged over the depth interval 40–80 m for Lakes Bourget and Geneva) for 2020–2099 for RCP 2.6 (blue) and RCP 8.5 (red). Anomalies were computed for each GCM based on departure from the historical reference period (1976–2005), and an ensemble GCM mean (thick line) with spread amongst the individual models (shaded area) is shown here for each lake. Winter defined here as November–February
The projected end-of-century lake winter temperature increases manifested in larger positive ADD anomalies with increasing radiative forcing under each RCP scenario (Fig. 6; Table 1). For all future emission scenarios, an increased number of ADD per winter were unanimously projected across all lakes. Under RCP 2.6, an average of + 113 ADD is expected across lakes, and under RCP 6.0, this would rise to + 212 ADD. Under the most severe warming scenario RCP 8.5, an average future winter in the period 2070–2099 across each lake is anticipated to have an extra 324 ADD, ranging from + 236.5 in Bunaveela to + 379.4 ADD for Geneva. Using the daily mean water temperature timeseries from the 1980–2019 hindcast simulation to obtain accurate estimates of total ADD per winter for each lake, the mean ADD across lakes for a November–February contemporary winter period was 718 ADD (Table 1). An additional 236.5–379.4 ADD during winter would therefore represent a significant percentage increase in the heat accumulated by developing charr eggs in each population. Severity of future warming is projected to be more severe in the deeper spawning depths of the peri-alpine lake regions (40–80 m) compared to the projected warming at the shallower spawning depths of the Irish and English lake (both < 5 m) (Figs. 5, 6; Table 1).
Boxplots showing accumulated degree-day (ADD) winter anomalies at specific spawning depths (2 m for Bunaveela, 5 m for Windermere, and averaged over the depth interval 40–80 m for Lakes Bourget and Geneva) between the historical reference period (1975–2005) and the future simulation period (2070–2099) using an ensemble mean GCM value under a RCP 2.6, b 6.0, and c 8.5 for each lake. Winter defined here as November–February
4 Discussion
Within freshwater environments, water temperature has a fundamental influence on key aspects of salmonid physiology and behaviour, affecting growth rates (e.g. O’Gorman et al. 2016), life-history phenology (e.g. Sparks et al. 2018), fecundity and reproduction (Elliott and Elliott 2010), predator–prey interactions (e.g. Bell et al. 2017), and determination of the spatial extent of useable habitat (e.g. Ebersole et al. 2001). Arctic charr populations at the southern periphery of their native range in Europe have declined significantly and in many lakes have undergone local extirpations, which has previously been attributed to multiple stressors including water quality, spawning habitat degradation, and non-native fish species (e.g. Maitland et al. 2007). Whilst climate change has been implicated as a possible contributor to declining Arctic charr populations (e.g. Winfield et al. 2010; Gerdeaux 2011), here we show definitive coherent warming trends during the critical winter season at spawning site depths in lakes currently inhabited by Arctic charr populations at the southernmost limit of their natural range. These freshwater resident Arctic charr populations may be especially vulnerable to climatic change, particularly in fragmented freshwater landscapes without the option to migrate to alternative nearby habitats that could provide a more suitable thermal refuge. Furthermore, the spawning and embryonic life stage are restricted to remaining in a very specific habitat type (rocky, gravelly substrate), meaning that temperature changes in these areas during winter will be largely unavoidable.
At the current rate of warming detected in the hindcast trend analysis, the max annual winter water temperature being reached is on average 1.1 °C warmer and the mean winter water temperature is on average 1.0 °C warmer compared to 40 years prior across our modelled lakes. Similar trends of lake winter temperatures warming at an accelerated rate relative to summer have been previously identified for European lakes (e.g. Woolway et al. 2019). Whilst high-productivity summer months may seem like the most obvious period for biologically relevant warming impacts to occur in lakes, here we draw attention to the possible consequences of winter warming on coldwater lake fish, particularly the salmonids, which spawn during the winter months. For example, a proportion of the adult Atlantic salmon (Salmo salar) that have returned from their marine migration also utilise streams fed by water from Bunaveela as spawning ground and could also ultimately be affected by warmer winters (McGinnity et al. 2009).
The reported thermal requirements of charr embryos are an excellent focus for highlighting the ontogenetically related physiological stresses that warmer winters might pose. The narrower temperature tolerance of the egg stage is related to a reduced aerobic capacity (and ability to supply oxygen to tissues), which improves from egg to adult with the development of the cardiorespiratory system (e.g. Dahlke et al. 2020). Charr eggs also have immature homeostatic capacities to tolerate adverse thermal conditions. For example, Lake Geneva charr eggs from a captive broodstock incubated at 8.5 °C showed low survival rates compared to those incubated at 5 °C, and the surviving eggs were generally of much smaller size and poorer quality (Mari et al. 2016). Thus, the finding that the past six winters have consistently had a number of winter days (average of 26 days) with temperatures in exceedance of 8.5 °C in Lake Geneva at 40-m depth where charr spawning sites are located is startling, as prior to 1990 in our study period, there was no evidence of winter water temperatures exceeding this threshold. Despite the significant water quality recovery that has occurred in Geneva over the same time period, a self-sustaining Arctic charr population does not appear to have been achieved (Caudron et al. 2014). The recent warming trend illustrated here is likely to be a contributing factor, particularly with respect to its plausible impact on spawning success. It should be noted that the hydrodynamic model utilised here provided a 1-D representation of the lake, and in Lake Geneva, some spatial variability in temperature at specific depth zones may occur, for example, if spawning sites are located in the vicinity of groundwater inflows. In Windermere’s south basin, Arctic charr numbers have declined steadily over the past four decades, and although eutrophication of the water may be implicit in this decline (Winfield et al. 2008; Winfield et al. 2015), our finding that every winter since 1995 has consistently had prolonged, uninterrupted periods (often in excess of 20 or 30 days) with water temperatures in exceedance of 8.5 °C is likely to be an additional contributing factor in reducing the survival of some charr eggs. In Bunaveela, little is known about changes in the size of the charr population over the past four decades, and given that eutrophication, non-native species, and fishing pressure are not major concerns in this lake, it represents an excellent testbed to discern how continued winter warming may affect the remaining charr population. Finally, in Lake Bourget, it was apparent that temperatures as high as 8.5 °C had not been reached at any of the specific spawning depths examined here. However, the significant warming trends that have already occurred in mean and max winter temperatures in combination with future warming projections would imply that such adverse temperatures being reached are a likely eventuality; for example, winter temperatures exceeded 8 °C at 40 m and 7 °C at 60 m for the first time in the 40-year period during 2018 (Fig. 3a).
Previous studies of Arctic charr in temperate regions have identified ‘deep’ lakes as suitable habitat (Klemetsen et al. 2003). Results here have shown that in Lake Geneva during November and December, temperatures at 40 m have already exceeded 8.5 °C in recent years, whilst at 60 and 80 m, this high threshold has not been reached. Depths of 40–80 m in Bourget also still appear to provide charr with reasonable temperatures for spawning and embryonic development. Thus, deeper spawning sites may continue to offer cool refugia for spawning charr, particularly as warming trends continue to rise. However, in spite of cooler temperatures, deeper spawning sites are appropriate only if oxygen concentrations and sediment substrate are suitable for embryonic development and emergent larvae. Climate change is anticipated to modify mixing regimes in lakes, with deep lakes particularly susceptible to a reduction in the frequency and extent of complete deep water mixing during winter (e.g. Woolway and Merchant 2019). Such a phenomenon has already been observed in other peri-alpine lakes during mild winters (Rempfer et al. 2010). This could lead to increased oxygen depletion in the deepest spawning depth zones as well as periods of accelerated warming rates compared to shallow lakes that continue to undergo complete mixing each winter. For example, the future warming projections at depths of 40–80 m in Lakes Bourget and Geneva indicated more severe warming compared to the shallow depths examined in Windermere and Lough Bunaveela (Figs. 5 and 6).
In Windermere, separate spawning events peak in November and February; results here have shown that in November, temperatures in exceedance of 8.5 °C have consistently been reached in the past 40 years, whilst in February, this temperature has not been approached, implying that the scarcer spring spawners may be less vulnerable to contemporary climate change. However, the warmer winter temperatures preceding the spring spawning event may interfere with ovulation in females, by delaying the timing of spawning and reducing egg viability or in some instances (e.g. temperatures > 11 °C) inhibiting ovulation entirely (Gillet 1991). Of concern therefore is that winter temperatures exceeding 12 °C have been reached in Windermere in the past two decades (Fig. 3d). In shallow Lough Bunaveela, water temperatures are isothermal with depth during winter, and with maximum winter temperatures frequently surpassing 11 °C in recent years and projected to continue rising, it highlights that climate change does represent a serious threat to Irish charr populations.
It is clear from this study that there is an imminent threat due to rising winter water temperatures to the viability of many relict Arctic charr populations found presently at southern latitudes. Individual charr populations are locally adapted, with behavioural and physiological adaptations to deal with variations in historical and contemporary winter temperature regimes such as spawning at different times, having different physiological temperature tolerances and population-specific ADD requirements for ova development. However, results from all four GCMs and three future emission scenarios show that spawning charr and developing embryos across all study lakes will invariably experience increased winter temperatures and ADDs per winter season by the end of the current century. The future temperature anomalies under RCP 8.5 are perhaps most pertinent, as despite its inclusion as a ‘worst-case’ scenario, RCP 8.5 emissions are within 1% accuracy of historical global emissions in the period 2006–2020. Thus, this presents a highly plausible scenario by 2100 (Schwalm et al. 2020). Under this scenario, temperatures will continue to rise over the course of the twenty-first century and beyond, with warming of 3–4 °C being reached by 2100 (Fig. 5). This could see mean winter temperatures in the range 9.5–10 °C in Lakes Bourget and Geneva (over the 40–80-m spawning depth range) and in the range 13–13.5 °C in Lough Bunaveela and Windermere. Spawning in female charr has been shown to be inhibited entirely at temperatures above 11 °C (Gillet 1991). An average November–February period during 2070–2099 will have an additional ~ 324 ADD, compared to contemporary thermal conditions of ~ 718 total ADD for the entire winter period (Table 1). Increases as large as 324 ADD will inevitably lead to modifications in the length of the incubation period and the viability and size of surviving eggs and subsequent emergent larvae (e.g. Gillet 1991; Mari et al. 2016). For example, typical charr egg incubation times before hatching is 400–500 ADD, although this depends on the specific population as well as the overall mean incubation temperature (e.g. Swift 1965; Mari et al. 2016). Overall, our results predict that a large increase in temperature accumulated by charr embryos will occur under future climatic conditions. However, further population-specific analyses are warranted in order to evaluate how changing temperatures over the course of the winter period, particularly at key developmental stages such as spawning and hatching, could represent a significant thermal stress and possibly lead to increased mortality rates and phenological changes. Changes in timing of spawning and early life-history phenology could lead to trophic mismatches and could disturb whole-lake food webs (e.g. Jonsson and Setzer 2015).
5 Conclusion
The findings of this study quantify for the first time the winter warming that has already occurred over a contemporary 40-year climatic period in Western and Central European lakes that currently provide refugia for Arctic charr at the southern periphery of their natural distribution. The total number of days during the spawning period with water temperatures exceeding temperatures that expose charr eggs to adverse thermal conditions has risen in three out of four of our study lakes. Winter warming is anticipated to continue unabated, which could ultimately inhibit spawning success in these regions entirely. The consequences of warming water temperatures could be compounded by local additional stressors that impact Arctic charr populations, including non-native fish species, suitable sediment substrate, and disease risk (e.g. Marcos-López et al. 2010; Winfield et al. 2015; Mari et al. 2016). The scope and severity of threats facing the temperate latitude Arctic charr epitomise the current crisis of biodiversity loss in freshwater ecosystems (Reid et al. 2019). With several extirpations previously documented in other lakes of these regions (Maitland et al. 2007), the additional threat of a rapidly warming climate may be sufficient to seal the fate of many peripheral Arctic charr populations.
Data availability
The ERA5 hourly data on single-level climate reanalysis dataset is available online at https://cds.climate.copernicus.eu/. The ISIMIP2b climate scenarios for each GCM under different RCPs are available online at https://www.isimip.org/gettingstarted/data-access/. The STOREFISH database can be accessed at http://storefish.org/. The GOTM configuration files for each lake are available at https://github.com/S-O-Ceallaigh/European-LakeCharr. The observed Lough Bunaveela temperature profiles (2009–2019) are available for download at https://github.com/S-O-Ceallaigh/European-LakeCharr. Observed temperature profiles from Lakes Bourget and Geneva are available from https://si-ola.inra.fr/si_lacs/login.jsf. Observed Windermere temperature profiles are available from https://doi.org/10.5285/bd37710e-6a53-49f0-9a07-6973408a3342. The 1980–2019 simulated temperature profiles and the future simulated temperature profiles for each lake are available from the corresponding author.
References
Bell DA, Kovach RP, Vulstek SC, Joyce JE, Tallmon DA (2017) Climate-induced trends in predator–prey synchrony differ across life-history stages of an anadromous salmonid. Can J Fish Aquat Sci 74:1431–1438. https://doi.org/10.1139/cjfas-2016-0309
Burchard H, Bolding K (2001) Comparative analysis of four second-moment turbulence closure models for the oceanic mixed layer. J Phys Oceanogr 31:1943–1968. https://doi.org/10.1175/1520-0485(2001)031<1943:CAOFSM>2.0.CO;2
Burchard H, Bolding K, Villarreal, M (1999) GOTM, a general ocean turbulence model: Theory, implementation and test cases. Luxembourg Rep. EUR 18745, 103 pp.
Butler JRA, Radford A, Riddington G, Laughton R (2009) Evaluating an ecosystem service provided by Atlantic salmon, sea trout and other fish species in the River Spey, Scotland: the economic impact of recreational rod fisheries. Fish Res 96:259–266. https://doi.org/10.1016/j.fishres.2008.12.006
Cachera S, Caudron A, Champigneulle A (2010) A study of the contribution of stocking and natural recruitment in the fisheries for Arctic charr (Salvelinus alpinus) and whitefish (Coregonus lavaretus) in Lake Bourget under reoligotrophication. In: Journées Internationales de Limnologie, Thonon-les-Bain, France, pp 63
Carter JL, Schindler DE, Francis TB (2017) Effects of climate change on zooplankton community interactions in an Alaskan lake. Clim Change Responses 4:3. https://doi.org/10.1186/s40665-017-0031-x
Caudron A, Lasne E, Gillet C, Guillard J, Champigneulle A (2014) Thirty years of reoligotrophication do not contribute to restore self-sustaining fisheries of Arctic charr, Salvelinus alpinus, in Lake Geneva. Fish Res 154:165–171. https://doi.org/10.1016/j.fishres.2014.01.023
Connor L, Shephard S, Rocks K, Kelly FL (2019) Potential climate change impacts on Arctic charr Salvelinus alpinus L. in Ireland. Fish Manag Ecol 26:527–539. https://doi.org/10.1111/fme.12327
Crisp DT (1996) Environmental requirements of common riverine European salmonid fish species in fresh water with particular reference to physical and chemical aspects. Hydrobiologia 323:201–221. https://doi.org/10.1007/BF00007847
Dahlke FT, Wohlrab S, Butzin M, Pörtner H-O (2020) Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369:65–70. https://doi.org/10.1126/science.aaz3658
Ebersole JL, Liss WJ, Frissell CA (2001) Relationship between stream temperature, thermal refugia and rainbow trout Oncorhynchus mykiss abundance in arid-land streams in the Northwestern United States. Ecol Freshw Fish 10:1–10. https://doi.org/10.1034/j.1600-0633.2001.100101.x
Elliott JM, Elliott JA (2010) Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. J Fish Biol 77:1793–1817. https://doi.org/10.1111/j.1095-8649.2010.02762.x
Fairall CW, Bradley EF, Rogers DP, Edson JB, Young GS (1996) Bulk parameterization of air-sea fluxes for tropical ocean-global atmosphere coupled-ocean atmosphere response experiment. J Geophys Res Oceans 101:3747–3764. https://doi.org/10.1029/95JC03205
Farmer TM, Marschall EA, Dabrowski K, Ludsin SA (2015) Short winters threaten temperate fish populations. Nat Commun 6:7724. https://doi.org/10.1038/ncomms8724
Frieler K, Lange S, Piontek F et al (2017) Assessing the impacts of 1.5 °C global warming–simulation protocol of the Inter-Sectoral Impact Model Intercomparison Project (ISIMIP2b). Geosci Model Dev 10:4321–4345. https://doi.org/10.5194/gmd-10-4321-2017
Gerdeaux D (2011) Does global warming threaten the dynamics of Arctic charr in Lake Geneva? Hydrobiologia 660:69–78. https://doi.org/10.1007/s10750-010-0412-7
Gillet C (1991) Egg production in an Arctic charr (Salvelinus alpinus L.) brood stock: effects of temperature on the timing of spawning and the quality of eggs. Aquat Living Resour 4:109–116. https://doi.org/10.1051/alr:1991010
Hempel S, Frieler K, Warszawski L, Schewe J, Piontek F (2013) A trend-preserving bias correction-the ISIMIP approach. Earth Syst Dyn 4:219–236. https://doi.org/10.5194/esd-4-219-2013
Hersbach H, Bell B, Berrisford P, Hirahara S, Horányi A, Muñoz-Sabater J, Nicolas J, Peubey C, Radu R, Schepers D, Simmons A, Soci C, Abdalla S, Abellan X, Balsamo G, Bechtold P, Biavati G, Bidlot J, Bonavita M, Chiara GD, Dahlgren P, Dee D, Diamantakis M, Dragani R, Flemming J, Forbes R, Fuentes M, Geer A, Haimberger L, Healy S, Hogan RJ, Hólm E, Janisková M, Keeley S, Laloyaux P, Lopez P, Lupu C, Radnoti G, de Rosnay P, Rozum I, Vamborg F, Villaume S, Thépaut J-N (2020) The ERA5 global reanalysis. Q J R Meteorol Soc 146:1999–2049. https://doi.org/10.1002/qj.3803
Hilborn R, Quinn TP, Schindler DE, Rogers DE (2003) Biocomplexity and fisheries sustainability. PNAS 100:6564–6568. https://doi.org/10.1073/pnas.1037274100
Humpesch UH (1985) Inter- and intra-specific variation in hatching success and embryonic development of five species of salmonids and Thymallus thymallus. Arch Hydrobiol 104:129–144
Igoe F, Hammar J (2004) The Arctic charr Salvelinus alpinus (L.) species complex in Ireland: a secretive and threatened ice age relict. Biol Environ 104B:73–92
Jankowski T, Livingstone DM, Bührer H, Forster R, Niederhauser P (2006) Consequences of the 2003 European heat wave for lake temperature profiles, thermal stability, and hypolimnetic oxygen depletion: implications for a warmer world. Limnol Oceanogr 51:815–819. https://doi.org/10.4319/lo.2006.51.2.0815
Jonsson T, Setzer M (2015) A freshwater predator hit twice by the effects of warming across trophic levels. Nat Commun 6:1–9. https://doi.org/10.1038/ncomms6992
Joy PJ, Stricker CA, Ivanoff R, Wipfli MS, Seitz AC, Tyers M (2020) Bridging the gap between Salmon Spawner abundance and marine nutrient assimilation by juvenile salmon: seasonal cycles and landscape effects at the watershed scale. Ecosystems 23:338–358
Jungwirth M, Winkler H (1984) The temperature dependence of embryonic development of grayling (Thymallus thymallus), Danube salmon (Hucho hucho), Arctic charr (Salvelinus alpinus) and brown trout (Salmo trutta fario). Aquaculture 38:315–327. https://doi.org/10.1016/0044-8486(84)90336-3
Kerr SJ, Grant RE (2000) Ecological impacts of fish introductions: evaluating the risk. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario 473 p
Klemetsen A, Amundsen P-A, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecology of Freshwater Fish 12:1–59. https://doi.org/10.1034/j.1600-0633.2003.00010.x
Lange S (2019) EartH2Observe, WFDEI and ERA-Interim data merged and bias-corrected for ISIMIP (EWEMBI). V.1.1. GFZ Data Services. https://doi.org/10.5880/pik.2019.004. Accessed 18-04-2019. (2019)
Livingstone DM (2003) Impact of secular climate change on the thermal structure of a large temperate Central European lake. Clim Chang 57:205–225. https://doi.org/10.1023/A:1022119503144
Low JJ, Igoe F, Davenport J, Harrison SSC (2011) Littoral spawning habitats of three southern Arctic charr (Salvelinus alpinus L.) populations. Ecol Freshw Fish 20:537–547. https://doi.org/10.1111/j.1600-0633.2011.00502.x
Maitland PS, Winfield IJ, McCarthy ID, Igoe F (2007) The status of Arctic charr Salvelinus alpinus in Britain and Ireland. Ecol Freshw Fish 16:6–19. https://doi.org/10.1111/j.1600-0633.2006.00167.x
Marcos-López M, Gale P, Oidtmann BC, Peeler EJ (2010) Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transbound Emerg Dis 57:293–304. https://doi.org/10.1111/j.1865-1682.2010.01150.x
Mari L, Garaud L, Evanno G, Lasne E (2016) Higher temperature exacerbates the impact of sediments on embryo performances in a salmonid. Biol Lett 12:20160745. https://doi.org/10.1098/rsbl.2016.0745
McGinnity P, Jennings E, de Eyto E, Allott N, Samuelsson P, Rogan G, Whelan K, Cross T (2009) Impact of naturally spawning captive-bred Atlantic salmon on wild populations: depressed recruitment and increased risk of climate-mediated extinction. Proc R Soc B Biol Sci 276:3601–3610. https://doi.org/10.1098/rspb.2009.0799
Miller H, Winfield IJ, Fletcher JM, James JB, Rijn J van, Bull JM, Cotterill CJ (2015) Distribution, characteristics and condition of Arctic charr (Salvelinus alpinus) spawning grounds in a differentially eutrophicated twin-basin lake. Ecology of Freshwater Fish 24:32–43. https://doi.org/10.1111/eff.12122
O’Gorman EJ, Ólafsson ÓP, Demars BOL, Friberg N, Guðbergsson G, Hannesdóttir ER, Jackson MC, Johansson LS, McLaughlin ÓB, Ólafsson JS, Woodward G, Gíslason GM (2016) Temperature effects on fish production across a natural thermal gradient. Glob Chang Biol 22:3206–3220. https://doi.org/10.1111/gcb.13233
O’Reilly CM, Sharma S, Gray DK et al (2015) Rapid and highly variable warming of lake surface waters around the globe. Geophys Res Lett 42:10,773–10,781. https://doi.org/10.1002/2015GL066235
Piggins DJ, Went AEJ (1971) Introduction of Arctic charr (Salvelinus alpinus) into Irish waters. Irish Nat J 17:144–144
Reavie ED, Sgro GV, Estepp LR, Bramburger AJ, Chraïbi VLS, Pillsbury RW, Cai M, Stow CA, Dove A (2017) Climate warming and changes in Cyclotella sensu lato in the Laurentian Great Lakes. Limnol Oceanogr 62:768–783. https://doi.org/10.1002/lno.10459
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Rempfer J, Livingstone DM, Blodau C, Forster R, Niederhauser P, Kipfer R (2010) The effect of the exceptionally mild European winter of 2006-2007 on temperature and oxygen profiles in lakes in Switzerland: a foretaste of the future? Limnol Oceanogr 55:2170–2180. https://doi.org/10.4319/lo.2010.55.5.2170
Rimet F, Anneville O, Barbet D, Charrdon C, Crépin L, Domaizon I, Dorioz J-M, Espinat L, Frossard V, Guillard J, Goulon C, Hamelet V, Hustache J-C, Jacquet S, Lainé L, Montuelle B, Perney P, Quetin P, Rasconi S, Schellenberger A, Tran-Khac V, Monet G (2020) The observatory on LAkes (OLA) database: sixty years of environmental data accessible to the public. J Limnol 79. https://doi.org/10.4081/jlimnol.2020.1944
Rubin JF, Buttiker B (1992) Les sites de fraye de l’omble chevalier (Salvelinus alpinus L.) dans le Léman. Bull Fr Pêche Piscic:69–82. https://doi.org/10.1051/kmae:1992015
Savary R, Dufresnes C, Champigneulle A, Caudron A, Dubey S, Perrin N, Fumagalli L (2017) Stocking activities for the Arctic charr in Lake Geneva: genetic effects in space and time. Ecol Evol 7:5201–5211. https://doi.org/10.1002/ece3.3073
Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS (2010) Population diversity and the portfolio effect in an exploited species. Nature 465:609–612. https://doi.org/10.1038/nature09060
Schneider P, Hook SJ (2010) Space observations of inland water bodies show rapid surface warming since 1985. Geophys Res Lett 37. https://doi.org/10.1029/2010GL045059
Schwalm CR, Glendon S, Duffy PB (2020) RCP8.5 tracks cumulative CO2 emissions. PNAS. https://doi.org/10.1073/pnas.2007117117
Shatwell T, Thiery W, Kirillin G (2019) Future projections of temperature and mixing regime of European temperate lakes. Hydrol Earth Syst Sci 23:1533–1551. https://doi.org/10.3929/ethz-b-000334578
Sparks MM, Falke JA, Quinn TP, Adkison MD, Schindler DE, Bartz K, Young D, Westley PAH (2018) Influences of spawning timing, water temperature, and climatic warming on early life history phenology in western Alaska sockeye salmon. Can J Fish Aquat Sci 76:123–135. https://doi.org/10.1139/cjfas-2017-0468
Swift DR (1965) Effect of temperature on mortality and rate of development of the eggs of the Windermere charr (Salvelinus alpinus). J Fish Res Bd Can 22:913–917. https://doi.org/10.1139/f65-086
Teletchea F, Fostier A, Le Bail P-Y, Jalabert B, Gardeur J-N, Fontaine P (2007) STOREFISH: a new database dedicated to the reproduction of temperate freshwater teleost fishes. Cybium 31:227–235
van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F, Masui T, Meinshausen M, Nakicenovic N, Smith SJ, Rose SK (2011) The representative concentration pathways: an overview. Clim Chang 109:5. https://doi.org/10.1007/s10584-011-0148-z
Whiteley AR, Penaluna BE, Taylor EB, Weiss S, Abadia-Cardoso A, Gomez-Uchida D, Koizumi I, Trotter P (2019) Trout and charr: taxonomy, systematics, and phylogeography. In: Kershner JL, Williams JE, Gresswell RE, Lobón-Cervia J (eds) Trout and charr of the world. Maryland, American Fisheries Society, pp 95–140
Winfield IJ, Fletcher JM, James JB (2008) The Arctic charr (Salvelinus alpinus) populations of Windermere, UK: population trends associated with eutrophication, climate change and increased abundance of roach (Rutilus rutilus). Environ Biol Fish 83:25–35. https://doi.org/10.1007/s10641-007-9235-4
Winfield IJ, Hateley J, Fletcher JM, James JB, Bean CW, Clabburn P (2010) Population trends of Arctic charr (Salvelinus alpinus) in the UK: assessing the evidence for a widespread decline in response to climate change. Hydrobiologia 650:55–65. https://doi.org/10.1007/s10750-009-0078-1
Winfield IJ, van Rijn J, Valley RD (2015) Hydroacoustic quantification and assessment of spawning grounds of a lake salmonid in a eutrophicated water body. Ecol Inform 30:235–240. https://doi.org/10.1016/j.ecoinf.2015.05.009
Winfield IJ, Berry R, Iddon H (2019) The cultural importance and international recognition of the Arctic charr Salvelinus alpinus populations of Windermere, UK. Hydrobiologia 840:11–19. https://doi.org/10.1007/s10750-018-3814-6
Wood SN (2017) Generalized Additive Models: An Introduction with R. 2nd ed. CRC Press, New York, pp 496
Woolway RI, Merchant CJ (2019) Worldwide alteration of lake mixing regimes in response to climate change. Nat Geosci 12:271. https://doi.org/10.1038/s41561-019-0322-x
Woolway RI, Weyhenmeyer GA, Schmid M et al (2019) Substantial increase in minimum lake surface temperatures under climate change. Clim Chang 155:81–94. https://doi.org/10.1007/s10584-019-02465-y
Yvon-Durocher G, Hulatt CJ, Woodward G, Trimmer M (2017) Long-term warming amplifies shifts in the carbon cycle of experimental ponds. Nat Clim Chang 7:209–213. https://doi.org/10.1038/nclimate3229
Acknowledgements
The authors wish to acknowledge the technical staff who have collected the long-term temperature data from each lake in this study. We also thank two reviewers for comments that have improved this manuscript.
Code availability
The General Ocean Turbulence Model source code and instructions for compilation are freely available at https://gotm.net/. The parsac Python code for model calibration is freely available at https://github.com/BoldingBruggeman/parsac.
Funding
This work was carried out with the support of the Marine Institute. Seán Kelly was funded under the Marine Research Programme by the Irish Government (BEYOND 2020 PBA/FS/16/02). Phil McGinnity was also supported by the Marine Institute under the Marine Research Programme 2014–2020 (RESPI/FS/16/01). Tadhg Moore was funded by the WATExR project, which is part of ERA4CS, an ERA-NET initiated by JPI Climate, and funded by MINECO (ES), FORMAS (SE), BMBF (DE), EPA (IE), RCN (NO), and IFD (DK), with co-funding by the European Union (Grant Number: 690462).
Author information
Authors and Affiliations
Contributions
Conceptualisation: Seán Kelly; methodology: Seán Kelly, Tadhg Moore, Eleanor Jennings; formal analysis and investigation: Seán Kelly; project administration, supervision, and funding acquisition: Eleanor Jennings and Phil McGinnity; writing—original draft: Seán Kelly; writing—review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1388 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kelly, S., Moore, T.N., de Eyto, E. et al. Warming winters threaten peripheral Arctic charr populations of Europe. Climatic Change 163, 599–618 (2020). https://doi.org/10.1007/s10584-020-02887-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10584-020-02887-z