Abstract
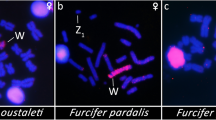
Among amniote vertebrates, geckos represent a clade with exceptional variability in sex determination; however, only a minority of species of this highly diverse group has been studied in this respect. Here, we describe for the first time a female heterogamety in the genus Paroedura, the group radiated in Madagascar and adjacent islands. We identified homomorphic ZZ/ZW sex chromosomes with a highly heterochromatic W chromosome in Paroedura masobe, Paroedura oviceps, Paroedura karstophila, Paroedura stumpffi, and Paroedura lohatsara. Comparative genomic hybridization (CGH) revealed that female-specific sequences are greatly amplified in the W chromosome of P. lohatsara and that P. gracilis seems to possess a derived system of multiple sex chromosomes. Contrastingly, neither CGH nor heterochromatin visualization revealed differentiated sex chromosomes in the members of the Paroedura picta—Paroedura bastardi—Paroedura ibityensis clade, which is phylogenetically nested within lineages with a heterochromatic W chromosome. As a sex ratio consistent with genotypic sex determination has been reported in P. picta, it appears that the members of the P. picta—P. bastardi—P. ibityensis clade possess homomorphic, poorly differentiated sex chromosomes and may represent a rare example of evolutionary loss of highly differentiated sex chromosomes. Fluorescent in situ hybridization (FISH) with a telomeric probe revealed a telomere-typical pattern in all species and an accumulation of telomeric sequences in the centromeric region of autosomes in P. stumpffi and P. bastardi. Our study adds important information for the greater understanding of the variability and evolution of sex determination in geckos and demonstrates how the geckos of the genus Paroedura provide an interesting model for studying the evolution of the sex chromosomes.





Similar content being viewed by others
Abbreviations
- CGH :
-
Comparative genomic hybridization
- DAPI :
-
4′,6-Diamidino-2-phenylindole
- DUTP:
-
Deoxy-uridine-5′-triphosphate
- ESD:
-
Environmental sex determination
- FISH:
-
Fluorescence in situ hybridization
- FITC:
-
Fluorescein isothiocyanate
- GSD:
-
Genotypic sex determination
- ITSs:
-
Interstitial telomeric sequences
- PCR:
-
Polymerase chain reaction
- SSC:
-
Saline sodium citrate
- SDS:
-
Sodium dodecyl sulfate
References
Aprea G, Andreone F, Fulgione D, Petraccioli A, Odierna G (2013) Chromosomal rearrangements occurred repeatedly and independently during species diversification in Malagasy geckos, genus Paroedura. Afr Zool 48:96–108
Bachtrog D (2008) The temporal dynamics of processes underlying Y chromosome degeneration. Genetics 119:1513–1525
Blumberg MS, Lewis SJ, Sokoloff G (2002) Incubation temperature modulates post-hatching thermoregulatory behavior in the Madagascar ground gecko, Paroedura pictus. J Exp Biol 205:2777–2784
Castiglia R, García A, Bezerra AMR, Flores-Villela O, Gornung E (2009) Karyotypic diversification due to Robertsonian rearrangements in Phyllodactylus lanei Smith, 1935 (Squamata, Gekkonidae) from Mexico. Rend Lincei 20:77–82
Cree A, Thompson MB, Daugherty CH (1995) Tuatara sex determination. Nature 375:543
De Oliveira RR, Feldberg E, dos Anjos MB, Zuanon J (2008) Occurrence of multiple sexual chromosomes (XX/XY1Y2 and Z1Z1Z2Z2/Z1Z2W1W2) in catfishes of the genus Ancistrus (Siluriformes: Loricariidae) from the Amazon basin. Genetica 134:243–249
Ezaz T, Quinn AE, Sarre SD et al (2009) Molecular marker suggests rapid changes of sex-determining mechanisms in Australian dragon lizards. Chromosome Res 17:91–98
Ferguson-Smith MA, Trifonov V (2007) Mammalian karyotype evolution. Nature Rev Genet 8:950–962
Folmer O, Black M, Hoeh WR et al (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gamble T (2010) A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex Dev 4:88–103
Gamble T, Geneva AJ, Glor RE, Zarkower D (2014) Anolis sex chromosomes are derived from a single ancestral pair. Evolution 68:1027–1041
Glaw F, Vences M (2007) A field guide to the amphibians and reptiles of Madagascar. Köln: Vences & Glaw Verlags GbR
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hawlitschek O, Glaw F (2013) The complex colonization history of nocturnal geckos (Paroedura) in the Comoros Archipelago. Zool Scr 42:135–150
Hebert PD, Gregory TR (2005) The promise of DNA barcoding for taxonomy. Syst Biol 54:852–859
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B Biol Sci 270:313–321
Ijdo JW, Wells RA, Baldini A, Reeders ST (1991) Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res 19:4780
Jackman TR, Bauer AM, Greenbaum E, Glaw F, Vences M (2008) Molecular phylogenetic relationships among species of the Malagasy-Comoran gecko genus Paroedura (Squamata: Gekkonidae). Mol Phylogenet Evol 46:74–81
Janzen FJ, Phillips PC (2006) Exploring the evolution of environmental sex determination, especially in reptiles. J Evol Biol 19:1775–1784
Jeong TJ, Jun J, Han S et al (2013) DNA barcode reference data for the Korean herpetofauna and their applications. Mol Ecol Resour 13:1019–1032
Kawai A, Ishijima J, Nishida C et al (2009) The ZW sex chromosomes of Gekko hokouensis (Gekkonidae, Squamata) represent highly conserved homology with those of avian species. Chromosoma 118:43–51
King M (1991) Chromosome change and speciation in lizards. In: Atchley WR, Woodruff DS, eds. Evolution and speciation. Cambridge University Press, pp 262–285
Kratochvíl L, Kubička L, Landová E (2008) Does the mechanism of sex determination constrain the potential for sex manipulation? A test in geckos with contrasting sex-determining systems. Naturwissenschaften 95:209–215
Kubička L, Starostová Z, Kratochvíl L (2012) Temperature-dependent rate of clutch production in a tropical lizard (Paroedura picta: Gekkonidae): intraspecific test of the metabolic theory of ecology. J Therm Biol 37:179–184
Lang JW, Andrews HV (1994) Temperature-dependent sex determination in crocodilians. J Exp Zool 270:28–44
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org
Main H, Scantlebury DP, Zarkower D, Gamble T (2012) Karyotypes of two species of Malagasy ground gecko (Paroedura: Gekkonidae). Afr J Herpetol 61:81–90
Matsubara K, Tarui H, Toriba M et al (2006) Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc Natl Acad Sci U S A 103:18190–18195
Matsubara K, Knopp T, Sarre SD et al (2013) Karyotypic analysis and FISH mapping of microsatellite motifs reveal highly differentiated XX/XY sex chromosomes in the pink-tailed worm-lizard (Aprasia parapulchella, Pygopodidae, Squamata). Mol Cytogenet 6:60
Murphy RW, Crawford AJ, Bauer AM et al (2013) Cold Code: the global initiative to DNA barcode amphibians and nonavian reptiles. Mol Ecol Resour 13:161–167
Nagy ZT, Sonet G, Glaw F, Vences M (2012) First large-scale DNA barcoding assessment of reptiles in the biodiversity hotspot of Madagascar, based on newly designed COI primers. PLoS ONE 7:e34506
Nishida-Umehara C, Tsuda Y, Ishijima J et al (2007) The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds. Chromosome Res 15:721–734
Noro M, Uejima A, Abe G, Manabe M, Tamura K (2009) Normal developmental stages of the Madagascar ground gecko Paroedura pictus with special reference to limb morphogenesis. Dev Dyn 238:100–109
O’Meally D, Patel HR, Stiglec R et al (2010) Non-homologous sex chromosomes of birds and snakes share repetitive sequences. Chromosome Res 18:787–800
Perrin N (2009) Sex reversal: a fountain of youth for sex chromosomes? Evolution 63:3043–3049
Pokorná M, Kratochvíl L (2009) Phylogeny of sex-determining mechanisms in squamate reptiles: are sex chromosomes an evolutionary trap? Zool J Linn Soc 156:168–183
Pokorná M, Rábová M, Ráb P et al (2010) Differentiation of sex chromosomes and karyotypic evolution in the eye-lid geckos (Squamata: Gekkota: Eublepharidae), a group with different modes of sex determination. Chromosome Res 18:809–820
Pokorná M, Kratochvíl L, Kejnovský E (2011) Microsatellite distribution on sex chromosomes at different stages of heteromorphism and heterochromatinization in two lizard species (Squamata: Eublepharidae: Coleonyx elegans and Lacertidae: Eremias velox). BMC Genet 12:90
Pokorná M, Altmanová M, Kratochvíl L (2014a) Multiple sex chromosomes in the light of female meiotic drive in amniote vertebrates. Chromosome Res 22:35–44
Pokorná M, Rens W, Rovatsos M, Kratochvíl L (2014b) A ZZ/ZW sex chromosome system in the thick-tailed gecko (Underwoodisaurus milii; Squamata: Gekkota: Carphodactylidae), a member of the ancient gecko lineage. Cytogenet Genome Res 142:190–196
Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 13:93
Rovatsos M, Altmanová M, Pokorná M, Kratochvíl L (2014a) Conserved sex chromosomes across adaptively radiated Anolis lizards. Evolution, 68:2079–2085. doi: 10.1111/evo.12357
Rovatsos M, Pokorná M, Altmanová M, Kratochvíl L (2014b) Cretaceous park of sex determination: sex chromosomes are conserved across iguanas. Biol Lett 10:20131093
Sarre SD, Ezaz T, Georges A (2011) Transitions between sex-determining systems in reptiles and amphibians. Annu Rev Genomics Hum Genet 12:391–406
Starostová Z, Kubička L, Kratochvíl L (2010) Macroevolutionary pattern of sexual size dimorphism in geckos corresponds to intraspecific temperature‐induced variation. J Evol Biol 23:670–677
Starostová Z, Angilletta MJ, Kubička L, Kratochvíl L (2012) Thermal dependence of reproductive allocation in a tropical lizard. J Therm Biol 37:159–163
Starostová Z, Kubička L, Golinski A, Kratochvíl L (2013) Neither male gonadal androgens nor female reproductive costs drive development of sexual size dimorphism in lizards. J Exp Biol 216:1872–1880
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Tamura K, Stecher G, Peterson D et al (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thompson JD, Higgins DG, Gibson TJ (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Uetz P, Hošek J (eds.) (2014) The reptile database. Retrieved from http://www.reptile-database.org 4/3/2014.
Valenzuela N, Lance VA (2004) Temperature-dependent sex determination in vertebrates. Smithsonian Institute, Washington
Vicoso B, Bachtrog D (2013) Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 499:332–335
Vicoso B, Emerson JJ, Zektser Y, Mahajan S, Bachtrog D (2013) Comparative sex chromosome genomics in snakes: differentiation, evolutionary strata, and lack of global dosage compensation. PLoS Biol 11:e1001643
Weiser H, Starostová Z, Kubička L, Kratochvíl L (2012) Overlap of female reproductive cycles explains shortened interclutch interval in a lizard with invariant clutch size (Squamata: Gekkonidae: Paroedura picta). Physiol Biochem Zool 85:491–498
Acknowledgment
The authors would like to express their gratitude to Jan Červenka for his valuable assistance with animal handling, to Zuzana Majtánová for her assistance with CGH methodology, to Petr Ráb and Marie Rábová for their support and advice regarding the experimental component, to Christopher Johnson for his linguistic improvements to the draft manuscript, and to two anonymous reviewers for their insightful comments. This project was supported by the Czech Science Foundation (GAČR 506/10/0718) and the Grant Agency of Charles University (GAUK 527112). This paper represents part 10 of our series “Evolution of sex-determining systems in lizards.”
Ethical standards
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fengtang Yang.
Rights and permissions
About this article
Cite this article
Koubová, M., Pokorná, M.J., Rovatsos, M. et al. Sex determination in Madagascar geckos of the genus Paroedura (Squamata: Gekkonidae): are differentiated sex chromosomes indeed so evolutionary stable?. Chromosome Res 22, 441–452 (2014). https://doi.org/10.1007/s10577-014-9430-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-014-9430-z