Abstract
Chromosome positioning at the equator of the mitotic spindle emerges out of a relatively entropic background. At this moment, termed metaphase, all kinetochores have typically captured microtubules leading to satisfaction of the spindle-assembly checkpoint, but the cell does not enter anaphase immediately. The waiting time in metaphase is related to the kinetics of securin and cyclin B1 degradation, which trigger sister-chromatid separation and promote anaphase processivity, respectively. Yet, as judged by metaphase duration, such kinetics vary widely between cell types and organisms, with no evident correlation to ploidy or cell size. During metaphase, many animal and plant spindles are also characterized by a conspicuous “flux” activity characterized by continuous poleward translocation of spindle microtubules, which maintain steady-state length and position. Whether spindle microtubule flux plays a specific role during metaphase remains arguable. Based on known experimental parameters, we have performed a comparative analysis amongst different cell types from different organisms and show that spindle length, metaphase duration and flux velocity combine within each system to obey a quasi-universal rule. As so, knowledge of two of these parameters is enough to estimate the third. This trend indicates that metaphase duration is tuned to allow approximately one kinetochore-to-pole round of microtubule flux. We propose that the time cells spend in metaphase evolved as a quality enhancement step that allows for the uniform stabilization/correction of kinetochore-microtubule attachments, thereby promoting mitotic fidelity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aim of mitosis is to generate two identical cells. Because cellular organelles are typically very abundant or undergo mitotic fragmentation, random distribution is “accurate” enough to guarantee a fair balance. For instance, random distribution of 400 (or N) independent organelles results in an acceptable 5 % (or N−1/2) imbalance between daughter cells. This is not tolerable in the case of chromosome segregation, where addition or subtraction of a chromosome (a condition known as aneuploidy) will tip the balance towards a compromised survival of the karyotype (Sheltzer et al. 2011).
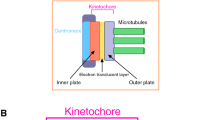
Chromosome segregation is mediated by a microtubule-based structure known as the mitotic spindle, which interacts with particular chromosomal interfaces called kinetochores (for reviews on molecular composition and structure, see Cheeseman and Desai 2008; DeLuca and Musacchio 2012; McEwen and Dong 2010). Kinetochore-spindle microtubule interactions are monitored by the spindle-assembly checkpoint (SAC), a signaling mechanism that prevents chromosome segregation in the presence of unattached kinetochores (Rieder et al. 1994; Rieder et al. 1995).
Several lines of evidence, notably from meiotic systems, suggest that chromosome positioning per se is not being monitored by the SAC (Gui and Homer 2012; Lane et al. 2012; Winey et al. 1995; Straight et al. 1997; Hays et al. 1982; Palevitz 1990). Yet, in many species, chromosomes do align at the cell equator, defining a state known as metaphase. The assembly of a metaphase plate motivates a quest for the meaning of metaphase and whether it represents an evolutionarily advantageous state (Nicklas and Arana 1992).
Chromosome alignment followed by their synchronous poleward movement appears to be a sensible strategy to ensure accurate chromosome segregation to the daughter cells, given that the presence of lagging chromosomes delays/prevents the completion of cytokinesis (Steigemann et al. 2009; Norden et al. 2006). Less obvious is the reason behind persistence in metaphase. Interestingly, cells from different organisms, as well as different cell types from the same organism, wait a highly different but characteristic time in metaphase. As astutely pointed out more than 50 years ago by Daniel Mazia, “We know practically nothing about what happens during the usual pause at metaphase except that something is happening!” (Mazia 1961).
In this review, we approach the problem of the metaphase state from an integrated perspective, with a focus on the role of kinetochore-microtubule interface plasticity. We argue that metaphase duration can be estimated from knowledge of the length and velocity scales that characterize metaphase spindle microtubules. This is supported by a literature survey (along with additional data provided by colleagues in the field and our own data) of relevant measurements on different cell types and species. We propose that the typical metaphase duration associated with any given system represents a quality enhancement step that, by allowing the formation of segregation-competent kinetochore-microtubule interfaces, increases the fidelity of mitosis.
Basic physical principles behind mitotic spindle mechanics
Metaphase can be viewed as a preparation for anaphase, where the relevance of kinetochore-spindle microtubule attachments is evidenced by the coordinated segregation of sister chromatids, kinetochores leading the way, before daughter cells are effectively separated during cytokinesis. The previous alignment of chromosomes at the equator of the forming mitotic spindle promotes a collective anaphase onset, with all chromosomes moving as a unit, a clearly more controllable situation. These observations, along with basic physical arguments, help in establishing a minimal set of conditions for a successful preparation and processivity of anaphase, which we outline below.
Forces that move chromosomes must be persistent and localized
Unlike a cannon ball, which moves due to forces applied to it in the past, a chromosome moves only if force is being applied to it (Fig. 1). This is not a particular property of chromosomes but of any object under high drag forces (Purcell 1977). Under such conditions, memory of applied forces quickly vanishes and mass (or inertia) becomes irrelevant compared with geometry (or drag). This central aspect of cellular mechanics demanded evolution of persistent force production mechanisms, capable of maintaining or recycling attachment to the pulled objects during motion. Although the origin of chromosomal drag in the cellular environment is multi-factorial, it is typically assumed that the phenomenological response is equivalent to a viscous drag, with velocity proportional to force.
Apart from making chromosomes particularly draggy, their size increases the probability of chromosomes to be subjected to multiple (eventually counteracting) forces. In most systems, unambiguous definition of the direction of motion is simplified by the fact that only a comparatively small portion of the chromatid body, the kinetochore, is devoted to energy coupling. As shown in Fig. 2 (see also Khodjakov and Rieder 1996; Carlson 1938), chromosome fragments which lack a kinetochore are effectively unable to segregate in a deterministic manner. Hence, both persistent and kinetochore-based attachments to spindle microtubules are necessary conditions for chromosome segregation.
A closed-loop force network must be in place to move chromosomes
In addition to a strong persistent pulling mechanism, another challenge is to warrant that it is the chromosome that effectively moves, and not the puller, which therefore must be firmly anchored until the chromosome reaches its final position. From first principles, the anchor must be large (mass is irrelevant), so one could envision structures such as the endoplasmic reticulum or the cell cortex acting as putative anchors for chromosome movement (Zheng 2010). However, one should take into account that even a small object/structure can separate two large objects (e.g., chromosomes), given that it is strong enough and pulls the two large objects simultaneously and in different (e.g., opposite) directions (Fig. 3). Resorting to a more conceptual framework, this situation, by conferring full symmetry to the system, breaks down hierarchical relations, so that each of the two separating objects may be regarded as an anchor for the other.
A closed-loop force network is required to move chromosomes. In its absence, it is the puller that moves towards the chromosome. Two possible loops are shown: in the inner loop (black), the circuit closes through the arrays of interpolar microtubules while in the outer loop (gray), it does so via the cortex
The closed-loop force network is independent of centrosomes
Along with chromosomes, the mitotic spindle is deeply connected to segregation of centrosomes, outliers in the partitioning problem because of their scarcity (two per dividing cell). Centrosomes tackle the partitioning problem by taking advantage of the mitotic spindle they helped to assemble in order to undergo one-to-one segregation (Debec et al. 2010). Centrosomes have been mechanically implicated in mitosis as being responsible for force production on spindle microtubules, allowing for poleward chromosome motion and segregation. This notion of the centrosomal anchor can be discarded as it is clear that closed-loop conditions cannot be satisfied if centrosomes are not themselves anchored. Despite their potential roles in spindle assembly, centrosomes should not be regarded as essential components of a minimal mechanical spindle, as underlined by the fact that many systems (e.g., land plants, vertebrate oocytes, and planarians) perform normal division in their absence (Zhang and Dawe 2011; Doubilet and McKim 2007; Azimzadeh et al. 2012).
The mitotic spindle is a dynamic closed structure that couples all chromosomes
Under the light microscope, the mitotic spindle in metaphase appears to behave as a unitary body. Translational and rotational motions are typically whole-spindle kinetic properties, with relatively small internal movements. Among the latter, individual chromosome oscillations are the most prominent, but even these tend to fade away as the cell progresses deep into metaphase (Jaqaman et al. 2010).
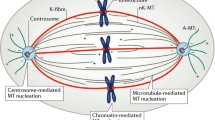
Advances in mitotic spindle modeling evolved from bulk observations to ever more detailed knowledge on structural, kinetic, and biochemical properties of the main spindle structures: microtubules (Margolis 1981; Mitchison and Kirschner 1984). Interestingly, the increasing knowledge on microtubule structure and behavior (polarity, polymeric nature, affinity to molecular motors, etc.) strengthened back the need for a collective approach to the spindle. In particular, the notion of a closed-loop force network dates back to late 1960s and to the first models which regarded non-kinetochore microtubules as more than microtubules that failed to attach to kinetochores (Fig. 3). Among these, McIntosh and colleagues proposed the “sliding filament” model by which motor-generated forces within the spindle might translate into motion in anaphase (McIntosh et al. 1969). This and subsequent works were probably the first to invoke some level of mechanical coupling between spindle elements, with a focus on lateral interactions between antiparallel interpolar microtubules and between parallel interpolar and kinetochore microtubules (Goode 1981; Margolis et al. 1978). The core message that arose from these studies was that anti-parallel overlapping microtubules were a convenient setting for polarity-sensitive motors to generate forces intrinsic to the spindle and, by the same mechanisms, that parallel microtubules would not promote relative motion but some degree of mechanical coupling. Thus, the mitotic spindle started to be regarded as a closed structure, with intrinsic properties dictating its own stability, symmetry, and scaling properties, paving the way for studies on spindle self-assembly in the following decades (Karsenti and Vernos 2001; Heald et al. 1996; Loughlin et al. 2011; Wuhr et al. 2008).
Spindle microtubule flux and kinetochore/centromere tension
Observation of kinetochore-microtubule attachments can reveal important aspects regarding the location of force generators. First, inter-kinetochore distance is increased in metaphase, suggesting that the centromeric region either is or was under tension. In the latter case, permanence of stretching might be explained by plastic deformation (or slow elastic recovery) of the centromere (Loncarek et al. 2007). However, experiments performed using microtubule depolymerizing drugs show that the centromere relaxes, at least partially, in the relevant timescale (minutes), supporting the notion that stretching effectively reports tension applied at that moment (Waters et al. 1996). Noteworthy, the kinetochore is itself stretched upon chromosome bi-orientation (Maresca and Salmon 2009; Uchida et al. 2009; Suzuki et al. 2011), an important observation which further suggests that the core mechanical connection between chromosomes and microtubules is not concentrated in the inner kinetochore region (DeLuca et al. 2005).
A second conspicuous aspect of metaphase spindles is the permanent motion of kinetochore-attached (as well as interpolar) microtubules away from the kinetochore (Fig. 4), a process termed microtubule poleward flux (Forer 1965; Hiramoto and Izutsu 1977; Bajer and Molè-Bajer 1972; Allen et al. 1969; Hamaguchi et al. 1987; Mitchison 1989). Flux, as an ordered translocation process, requires application of persistent forces. In addition, given that microtubule length and position are essentially maintained, such process requires permanent microtubule polymerization/depolymerization at kinetochores/poles to compensate for tubulin translocation (Fig. 4a).
Microtubule poleward flux. a Complementary (GTP dependent) polymerization dynamics at the microtubule tips leads to apparent translation of microtubules while, in fact, dimers are static (top). A plus end-directed motor can attach to a pair of anti-parallel (interpolar) microtubules and “move” towards both plus-ends simultaneously by sliding the microtubules apart. This ATP-dependent process produces real translocation of tubulin (middle). If both processes (polymerization dynamics and sliding) balance each other, as in poleward flux, then the microtubules tips are immobile but their components are effectively moving (bottom). Not shown is the equivalent process that occurs for kinetochore-attached microtubules, in which case the poleward force is likely not produced directly by motors but transferred from sliding interpolar microtubules to kinetochore microtubules. b Poleward flux can be directly observed when tubulin is non-uniformly labeled, so that reference dimers (as in (a)) can be used as fiduciary marks. Three superimposed frames of a Drosophila S2 mitotic spindle are shown, with RGB channels coding for time progression. Scale bar, 5 μm
Microtubule flux and kinetochore/centromere stretching, being simultaneous, indicate that the force underlying these two processes is extrinsic to the kinetochore. In fact, if the poleward force were generated at the kinetochore, the reaction force would tend to compress the kinetochore and decrease inter-kinetochore distance (Fig. 5).
Force that produces centromere/kinetochore tension and flux must be generated outside the kinetochore. a After amphitelic attachment, the centromere/kinetochores stretch due to tension while microtubules slip away from the kinetochore. b By the action-reaction principle, the positive correlation between tension and flux indicates that the underlying force is extrinsic to the kinetochore. A negative correlation between tension and flux would be observed for kinetochore-based force production mechanisms
Kinetochore/centromere stretching ceases when the elastic restoring force developed between kinetochores is balanced by the poleward force, while flux is persistent. Recalling that it is kinetochore/centromere tension, not flux, which is thought to be involved in stabilization of microtubule attachments, it seems somewhat paradoxical that the attachment sites let microtubules slip, for it reflects loss of tension. From this angle, a slippage/flux response to the poleward force appears to result from low kinetochore-microtubule affinity, a “weakness” which results in imperfect energy coupling between the poleward driving mechanisms (e.g., an ATP-dependent motor enzyme) and chromosomes. As a general prediction, given a poleward non-kinetochore force, flux and tension levels should have opposite trends upon modulation of the microtubule-kinetochore affinity.
The spindle-assembly checkpoint in metaphase
The metaphase plate is typically maintained for some minutes after the SAC requirements are satisfied. “Metaphase onset” is characterized by the triggering of a biochemical cascade, involving securin and cyclin B1 degradation which will lead, typically after some minutes, to sister chromatid disjunction and processive poleward motion, respectively (Clute and Pines 1999; Hagting et al. 2002). Within a cell type, metaphase duration displays only modest variability when compared with inter-cell type or inter-species variability (Meraldi et al. 2004) (Table 1).
Whether the SAC also monitors the ‘status’ of attachments (e.g., bi-orientation and establishment of tension) or blindly relies on efficient and timely correction mechanisms has been a matter of debate ever since (Pinsky and Biggins 2005; Lampson and Cheeseman 2011; Khodjakov and Rieder 2009; Nezi and Musacchio 2009; Itabashi et al. 2012; Khodjakov and Pines 2010). Naively, an “attachment-only SAC”, with tension playing critical but not directly monitored roles in promoting specific stabilization/correction of chromosome attachments, seems a more controllable situation, as it assigns a binary status to a single variable, with other parameters (e.g., tension) performing upstream modulation of that variable.
It is now well established that a tension-attachment link exists, with the former promoting stabilization/correction of the latter by a mechanism dependent on Aurora B (King and Nicklas 2000; Liu et al. 2009; Nicklas and Ward 1994; Ault and Nicklas 1989; Nicklas and Koch 1969; Lampson et al. 2004; Lampson and Cheeseman 2011). This by itself underlines the importance of tension and, consequently, of the spindle force map even when force is not translated into chromosome motion, as is the case during metaphase.
Coupling and the maturation of kinetochore-microtubule attachments
Although little is known about the particular architecture and state of mature kinetochore-microtubule attachments (e.g., how many microtubules must be interacting with the kinetochore? How deep? For how long?), the best available quantitative data suggest that anaphase onset is triggered at about 85 % of the maximal observed microtubule occupancy level at any given kinetochore (McEwen et al. 1997). Attachment maturation, as defined by the process leading to the formation of segregation-competent kinetochore-microtubule attachments, is a tension-dependent process in that tension increases microtubule occupancy and selectively stabilizes amphitelic attachments. Establishment of tension uniformity might therefore be important to promote unbiased error correction.
Tension uniformity may be attained by numerous mechanisms, which can be grouped in three main categories (Fig. 6a). One involves an active mechanism that reads out and compensates for differences in tension levels applied at kinetochores. The second category does not rely on sensors and exploits tension saturation. In this case, which would imply non-linear mechanical transduction, similar tension levels would be observed at kinetochores regardless of eventually different tensions being applied by the force-generating mechanisms. A third category relies on mechanical coupling of the different kinetochore-microtubule interfaces. By this mechanism, which is passive and independent of saturation, non-uniformity is an inherently unstable condition and the system will naturally evolve to equilibrium. Crucially, coupling-induced uniformity will only work if the elements to be equalized allow eventual drops in local tension, in a cooperative effort (although passive) to reach uniformity. In the spindle, this translates into a need to allow tension release, as is the case when kinetochore-microtubules slip/flux poleward. Regardless of the precise mechanisms behind its attainment, uniformity is always the best approach in spending (a finite amount of) energy if some threshold, even if unknown, is to be surpassed (Fig. 6b).
Coupling as a natural path towards uniformity. a A uniform distribution may be attained through measurement (blue), through saturation (green), or coupling (red). Coupling guarantees uniformity in a natural way, without resorting to sensors or saturation. b For any finite input, the efficiency of a system in reaching a threshold in a set of elements is maximized by uniformity
In addition to heuristic arguments, there is growing experimental evidence that the mitotic spindle is a viscoelastic-coupled structure mediated by microtubule crosslinking (Charlebois et al. 2011; Shimamoto et al. 2011; Itabashi et al. 2009). Putative mechanical couplers in the mitotic spindle include the variety of microtubule cross-linking proteins like NuMA, Dynein/Dynactin, HSET/kinesin-14, or Eg5/kinesin-5, which are known to establish connections between spindle microtubules (Walczak and Heald 2008). Also, it has been shown that microtubule conformational changes are strongly constrained by the surrounding cytoskeleton (Brangwynne et al. 2006). These suggest that local events of poleward microtubule translocation/flux likely have an impact on neighboring spindle microtubules.
According to the “coupled-spindle” model (Fig. 7a) proposed by Matos et al. (2009), spindle microtubule coupling is an essential condition for balanced force distribution while not requiring complex sensors and molecular feedback loops. As a corollary of this model, metaphase duration may be tuned to allow uniform maturation of the kinetochore-microtubule interfaces, so that anaphase typically does not begin before forces are balanced across the metaphase plate.
Flux and error correction in metaphase
As a general rule, chromosomes segregate accurately, suggesting that the catalytic timescales involved in cyclin B1 and securin degradation are typically large enough to allow the last chromosome(s) to correct eventual misalignments or attachment errors before cohesion between chromatids is effectively lost. Indeed, merotelic attachments are still detectable during metaphase (Knowlton et al. 2006) and their correction is promoted by experimentally increasing metaphase duration (Cimini et al. 2003), suggesting that error correction is still taking place. We call this a “blind clock” to stress that the catalytic timescales of cyclin B1 and securin degradation define an intrinsic timer. As depicted in Fig. 7b, a model invoking an intrinsic timer is consistent with the fact that metaphase duration is fairly predictable within a cell type. A consequence of a blind clock is that failure to correct attachment errors on time will lead to unbalanced partition of the genome between daughter cells (aneuploidy), instead of extending metaphase. Indeed, some merotelic attachments perdure and are eventually corrected only in anaphase (Cimini et al. 2004).
Coupling mechanics and chemistry of the metaphase waiting time. a The coupled-spindle model hypothesizes that mechanical coupling between spindle microtubules contributes to distribute force to and between kinetochores, the latter occurring only if kinetochores let microtubules slip away (i.e., flux) when subjected to the poleward force. Flux-mediated tension redistribution occurs in a timescale τ which is defined by the ratio between the spatial scale of spindle deformations (half-spindle length) and the velocity scale at which these are released (flux velocity). b In the “blind clock” model, metaphase duration is determined by the degradation kinetics of cyclin B/securin, and therefore is likely an intrinsic property of each cell type. Cells may enter anaphase before the typical time required to solve attachment problems. However, the frequency of these events can be decreased by evolutionary modulation of the clock pace and/or by modulation of the “twin” parameters, spindle length, and flux velocity
A relation between flux velocity, spindle size and metaphase duration
We previously proposed a model for the metaphase spindle which, by invoking mechanical coupling between spindle microtubules, predicted that equalization of tension along the metaphase plate could be achieved without measuring tension (Matos et al. 2009). Such passive mechanism would lend the system naturally to equilibrium, but only after some relaxation time, given by L/F, where L is a characteristic length scale of the relaxation process (half-spindle length) and F the velocity scale for the relaxation (flux velocity). Given our hypothesis that metaphase duration should be long enough to allow the system to relax, then metaphase duration, T, arises naturally as being correlated to the relaxation time, through T = c·L/F, where c, the cycle number, is a constant that should be expected not to differ significantly from 1. The reasoning is that c >> 1 represents many cycles of tension equalization before anaphase, an apparent waste of time at a time when the cell has halted or slowed down most of its ‘ordinary life’ mechanisms (e.g., transcription and migration), while c << 1 does not allow equalization to occur, promoting segregation errors. This notion of temporal optimization during mitosis is further supported by the observation that a transient mitotic delay that does not compromise completion of the process triggers a subsequent G1 arrest of the daughter cells (Uetake and Sluder 2010), whereas acceleration of the process (e.g., Mad2 depletion), even upon completion of chromosome alignment, results in massive missegregation (Matos et al. 2009).
To further test this model, we performed a quantitative analysis based on parameters found in the literature for different cell types and organisms (Table 1; Fig. 8). The predicted trend is generally observed, with metaphase duration increasing with the time necessary to achieve tension uniformity—relaxation time. Notably, two data points are clearly offset (Tobacco BY-2 cells and mouse oocytes (MI)) which were excluded from the fit in Fig. 8b, c, where the parameter c was estimated to be 1.1. We show in Fig. 8d the hyperboloid model function with all three parameters isolated, where again the data points combine to collapse to the model surface. The reasons behind the exceptional behavior in Tobacco BY-2 cells and mouse oocytes (MI) are unknown, but we speculate that it might be related with the fact that plants in general are more tolerant to aneuploidy (Matzke et al. 2003), while propagation of aneuploidy in the germline is normally prevented by loss of organism viability (Hassold and Hunt 2001). Given the general trend observed in somatic cells, two parameters are enough to estimate the third (Fig. 8e).
The coupled-spindle model across 15 cell types and organisms. a Definition of parameters. b Experimental data points (see Table 1). In the inset, number of flux-driven tubulin cycles in one metaphase time for each of the 15 organisms/cell types. c Linear fit to the data points with and without baseline, both yielding a slope of 1.1. The values for Tobacco BY-2 cells and Mouse oocytes were considered outliers and were not accounted for in fittings. d Representation of the data points but now with all three parameters isolated, along with the model surface. Each data point is shown connected to the closest point in the model surface by a black line
Finally, such tuning of spatio-temporal parameters seems not to occur at the “experimental timescale.” For example, modulation of flux velocity in the lab (Matos et al. 2009) does not impact metaphase duration, while it does so in the “evolutionary timescale.” These results further support that an intrinsic clock following SAC satisfaction is better adapted to the understanding of metaphase.
Conclusions
The problem of microtubule poleward flux (and of many aspects of the mitotic spindle) has been largely approached in a one-chromosome perspective, a scale at which it is hard to find an advantage for the kinetochore to let microtubules slide in response to poleward forces, for that leads to diminished tension. We reasoned that if a portion of the elastic energy released upon slippage were stored within the spindle, it might contribute to increase tension in other chromosomes, a process which would occur within a characteristic relaxation timescale.
By surveying the literature, we combined the three basic parameters involved (metaphase duration, flux velocity, and spindle length) in different cell types and species and found that experimental data fit reasonably well with the theoretical prediction. The biological message of the fitting is that metaphase lasts long enough to allow one cycle of flux-driven tubulin recycling in the spindle.
It is probably improper to define hierarchical relations among the three parameters, as this is likely an evolution-driven interplay. Existence of a hierarchy implies “knowledge of relative position” along the stream of events, which means “sensing”. This would be the case, for example, if the SAC waited for tension equalization, in which case we could say that metaphase duration is modulated by the combination of the two other parameters. It will be interesting to confirm if sensing is present—the “experiment timescale” hypothesis, or is indeed generally absent—the “evolutionary timescale” hypothesis. An interesting merge between these scales is found in those systems which have evolved to display very discrepant parameters in experimental timescales. For example, the first divisions of developing eggs (Wuhr et al. 2008), which display a very steep trend in spindle length, evolved for millions of years but are separated by minutes. The test then would be to check if subsequent divisions collapse to the model surface (Fig. 8c) but with different parameter combinations, i.e., in different locations of the hyperbolic surface of Fig. 8d.
Within the framework of the model, it is tempting to suggest that these results provide another piece of evidence for a tension-independent SAC. Failure to achieve a tension threshold will not prevent anaphase but it will be more error-prone, compromising progeny viability. Finally, we stress that even if the observed correlation may be explained by alternative models (e.g., a flux-driven kinetochore-to-pole transport model), what these results do certainly show is that poleward flux is a metaphase process and not merely a precociously activated anaphase-assisting mechanism.
Abbreviations
- ATP:
-
Adenosine triphosphate
- Mad2:
-
Mitotic arrest deficient 2
- NuMA:
-
Nuclear apparatus mitotic protein
- SAC:
-
Spindle-assembly checkpoint
References
Allen RD, Bajer A, Lafountain J (1969) Poleward migration of particles or states in spindle fiber filaments during mitosis in Haemanthus. J Cell Biol 43:4a
Amaro AC, Samora CP, Holtackers R, Wang E, Kingston IJ, Alonso M, Lampson M, McAinsh AD, Meraldi P (2010) Molecular control of kinetochore-microtubule dynamics and chromosome oscillations. Nat Cell Biol 12:319–329
Arnaoutov A, Azuma Y, Ribbeck K, Joseph J, Boyarchuk Y, Karpova T, McNally J, Dasso M (2005) Crm1 is a mitotic effector of Ran-GTP in somatic cells. Nat Cell Biol 7:626–632
Ault JG, Nicklas RB (1989) Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma 98:33–39
Azimzadeh J, Wong ML, Downhour DM, Sanchez Alvarado A, Marshall WF (2012) Centrosome loss in the evolution of planarians. Science 335:461–463
Bajer AS (1982) Functional autonomy of monopolar spindle and evidence for oscillatory movement in mitosis. J Cell Biol 93:33–48
Bajer AS, Molè-Bajer J (1972) Spindle dynamics and chromosome movements. Int Rev Cytol (Supplement 3):1–271
Brangwynne CP, Mackintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA (2006) Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 173:733–741
Brust-Mascher I, Scholey JM (2002) Microtubule flux and sliding in mitotic spindles of Drosophila embryos. Mol Biol Cell 13:3967–3975
Brust-Mascher I, Civelekoglu-Scholey G, Kwon M, Mogilner A, Scholey JM (2004) Model for anaphase B: role of three mitotic motors in a switch from poleward flux to spindle elongation. Proc Natl Acad Sci U S A 101:15938–15943
Brust-Mascher I, Sommi P, Cheerambathur DK, Scholey JM (2009) Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Mol Biol Cell 20:1749–1762
Buster DW, Zhang D, Sharp DJ (2007) Poleward tubulin flux in spindles: regulation and function in mitotic cells. Mol Biol Cell 18:3094–3104
Cai S, Weaver LN, Ems-Mcclung SC, Walczak CE (2009) Kinesin-14 family proteins HSET/XCTK2 control spindle length by cross-linking and sliding microtubules. Mol Biol Cell 20:1348–1359
Cameron LA, Yang G, Cimini D, Canman JC, Kisurina-Evgenieva O, Khodjakov A, Danuser G, Salmon ED (2006) Kinesin 5-independent poleward flux of kinetochore microtubules in PtK1 cells. J Cell Biol 173:173–179
Carlson JG (1938) Mitotic behavior of induced chromosomal fragments lacking spindle attachments in the neuroblasts of the grasshopper. Proc Natl Acad Sci U S A 24:500–507
Charlebois BD, Kollu S, Schek HT, Compton DA, Hunt AJ (2011) Spindle pole mechanics studied in mitotic asters: dynamic distribution of spindle forces through compliant linkages. Biophys J 100:1756–1764
Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9:33–46
Cimini D, Moree B, Canman JC, Salmon ED (2003) Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci 116:4213–4225
Cimini D, Cameron LA, Salmon ED (2004) Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr Biol 14:2149–2155
Clute P, Pines J (1999) Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol 1:82–87
Czaban BB, Forer A, Bajer AS (1993) Ultraviolet microbeam irradiation of chromosomal spindle fibres in Haemanthus katherinae endosperm. I. Behaviour of the irradiated region. J Cell Sci 105(Pt 2):571–578
Debec A, Sullivan W, Bettencourt-Dias M (2010) Centrioles: active players or passengers during mitosis? Cell Mol Life Sci 67:2173–2194
Deluca JG, Musacchio A (2012) Structural organization of the kinetochore-microtubule interface. Curr Opin Cell Biol 24:48–56
Deluca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED, McEwen BF (2005) Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell 16:519–531
Dhonukshe P, Vischer N, Gadella TW Jr (2006) Contribution of microtubule growth polarity and flux to spindle assembly and functioning in plant cells. J Cell Sci 119:3193–3205
Doubilet S, McKim KS (2007) Spindle assembly in the oocytes of mouse and Drosophila—similar solutions to a problem. Chromosome Res 15:681–696
Euteneuer U, Jackson WT, McIntosh JR (1982) Polarity of spindle microtubules in Haemanthus endosperm. J Cell Biol 94(3):644–653
Ferenz NP, Wadsworth P (2007) Prophase microtubule arrays undergo flux-like behavior in mammalian cells. Mol Biol Cell 18:3993–4002
Forer A (1965) Local reduction of spindle fiber birefringence in living Nephrotoma suturalis (Loew) spermatocytes induced by ultraviolet microbeam irradiation. J Cell Biol 25(SUPPL):95–117
Ganem NJ, Upton K, Compton DA (2005) Efficient mitosis in human cells lacking poleward microtubule flux. Curr Biol 15:1827–1832
Goode D (1981) Microtubule turnover as a mechanism of mitosis and its possible evolution. Biosystems 14:271–287
Goshima G, Wollman R, Stuurman N, Scholey JM, Vale RD (2005) Length control of the metaphase spindle. Curr Biol 15:1979–1988
Gui L, Homer H (2012) Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development 139:1941–1946
Hagting A, den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J (2002) Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol 157:1125–1137
Hamaguchi Y, Toriyama M, Sakai H, Hiramoto Y (1987) Redistribution of fluorescently labeled tubulin in the mitotic apparatus of sand dollar eggs and the effects of taxol. Cell Struct Funct 12:43–52
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280–291
Hayashi T, Sano T, Kutsuna N, Kumagai-Sano F, Hasezawa S (2007) Contribution of anaphase B to chromosome separation in higher plant cells estimated by image processing. Plant Cell Physiol 48:1509–1513
Hays TS, Wise D, Salmon ED (1982) Traction force on a kinetochore at metaphase acts as a linear function of kinetochore fiber length. J Cell Biol 93:374–389
Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E (1996) Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382:420–425
Hiramoto Y, Izutsu K (1977) Poleward movement of "markers" existing in mitotic spindles of grasshopper spermatocytes. Cell Struct Funct 2:257–259
Inoue S, Bajer A (1961) Birefringence in endosperm mitosis. Chromosoma 12:48–63
Itabashi T, Takagi J, Shimamoto Y, Onoe H, Kuwana K, Shimoyama I, Gaetz J, Kapoor TM, Ishiwata S (2009) Probing the mechanical architecture of the vertebrate meiotic spindle. Nat Methods 6:167–172
Itabashi T, Terada Y, Kuwana K, Kan T, Shimoyama I, Ishiwata S (2012) Mechanical impulses can control metaphase progression in a mammalian cell. Proc Natl Acad Sci U S A 109:7320–7325
Jaqaman K, King EM, Amaro AC, Winter JR, Dorn JF, Elliott HL, McHedlishvili N, McClelland SE, Porter IM, Posch M, Toso A, Danuser G, McAinsh AD, Meraldi P, Swedlow JR (2010) Kinetochore alignment within the metaphase plate is regulated by centromere stiffness and microtubule depolymerases. J Cell Biol 188:665–679
Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ (1998) Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol 141:1393–1406
Karsenti E, Vernos I (2001) The mitotic spindle: a self-made machine. Science 294:543–547
Khodjakov A, Pines J (2010) Centromere tension: a divisive issue. Nat Cell Biol 12:919–923
Khodjakov A, Rieder CL (1996) Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J Cell Biol 135:315–327
Khodjakov A, Rieder CL (2009) The nature of cell-cycle checkpoints: facts and fallacies. J Biol 8:88
Khodjakov A, Cole RW, Bajer AS, Rieder CL (1996) The force for poleward chromosome motion in Haemanthus cells acts along the length of the chromosome during metaphase but only at the kinetochore during anaphase. J Cell Biol 132:1093–1104
King JM, Nicklas RB (2000) Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J Cell Sci 113(Pt 21):3815–3823
Knowlton AL, Lan W, Stukenberg PT (2006) Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol 16:1705–1710
Kurihara D, Matsunaga S, Uchiyama S, Fukui K (2008) Live cell imaging reveals plant aurora kinase has dual roles during mitosis. Plant Cell Physiol 49:1256–1261
Lampson MA, Cheeseman IM (2011) Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol 21:133–140
Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM (2004) Correcting improper chromosome–spindle attachments during cell division. Nat Cell Biol 6:232–237
Lane SI, Yun Y, Jones KT (2012) Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development 139:1947–1955
Laycock JE, Savoian MS, Glover DM (2006) Antagonistic activities of Klp10A and Orbit regulate spindle length, bipolarity and function in vivo. J Cell Sci 119:2354–2361
Lee K, Kenny AE, Rieder CL (2010) P38 mitogen-activated protein kinase activity is required during mitosis for timely satisfaction of the mitotic checkpoint but not for the fidelity of chromosome segregation. Mol Biol Cell 21:2150–2160
Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB, Hoog C, Herbert M (2010) Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 20:1511–1521
Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM (2009) Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science 323:1350–1353
Loncarek J, Kisurina-Evgenieva O, Vinogradova T, Hergert P, la Terra S, Kapoor TM, Khodjakov A (2007) The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature 450:745–749
Loughlin R, Wilbur JD, McNally FJ, Nedelec FJ, Heald R (2011) Katanin contributes to interspecies spindle length scaling in Xenopus. Cell 147:1397–1407
Ma N, Tulu US, Ferenz NP, Fagerstrom C, Wilde A, Wadsworth P (2010) Poleward transport of TPX2 in the mammalian mitotic spindle requires dynein, Eg5, and microtubule flux. Mol Biol Cell 21:979–988
Maffini S, Maia AR, Manning AL, Maliga Z, Pereira AL, Junqueira M, Shevchenko A, Hyman A, Yates JR 3rd, Galjart N, Compton DA, Maiato H (2009) Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr Biol 19:1566–1572
Maresca TJ, Salmon ED (2009) Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol 184:373–381
Margolis RL (1981) Role of GTP hydrolysis in microtubule treadmilling and assembly. Proc Natl Acad Sci U S A 78:1586–1590
Margolis RL, Wilson L, Keifer BI (1978) Mitotic mechanism based on intrinsic microtubule behaviour. Nature 272:450–452
Matos I, Pereira AJ, Lince-Faria M, Cameron LA, Salmon ED, Maiato H (2009) Synchronizing chromosome segregation by flux-dependent force equalization at kinetochores. J Cell Biol 186:11–26
Matzke MA, Mette MF, Kanno T, Matzke AJ (2003) Does the intrinsic instability of aneuploid genomes have a causal role in cancer? Trends Genet 19:253–256
Mazia D (1961) Mitosis and the physiology of cell division. In: Brachet J, Mirsky AE (eds) The cell. Academic Press, New York
McCleland ML, Farrell JA, O'Farrell PH (2009) Influence of cyclin type and dose on mitotic entry and progression in the early Drosophila embryo. J Cell Biol 184:639–646
McEwen BF, Dong Y (2010) Contrasting models for kinetochore microtubule attachment in mammalian cells. Cell Mol Life Sci 67:2163–2172
McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL (1997) Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J Cell Biol 137:1567–1580
McIntosh JR, Hepler PK, van Wie DG (1969) Model for mitosis. Nature 224:659–663
Meraldi P, Draviam VM, Sorger PK (2004) Timing and checkpoints in the regulation of mitotic progression. Dev Cell 7:45–60
Minden JS, Agard DA, Sedat JW, Alberts BM (1989) Direct cell lineage analysis in Drosophila melanogaster by time-lapse, three-dimensional optical microscopy of living embryos. J Cell Biol 109:505–516
Mitchison TJ (1989) Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol 109:637–652
Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242
Mitchison TJ, Salmon ED (1992) Poleward kinetochore fiber movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J Cell Biol 119:569–582
Morales-Mulia S, Scholey JM (2005) Spindle pole organization in Drosophila S2 cells by dynein, abnormal spindle protein (Asp), and KLP10A. Mol Biol Cell 16:3176–3186
Nezi L, Musacchio A (2009) Sister chromatid tension and the spindle assembly checkpoint. Curr Opin Cell Biol 21:785–795
Nicklas RB, Arana P (1992) Evolution and the meaning of metaphase. J Cell Sci 102(Pt 4):681–690
Nicklas RB, Koch CA (1969) Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J Cell Biol 43:40–50
Nicklas RB, Ward SC (1994) Elements of error correction in mitosis: microtubule capture, release, and tension. J Cell Biol 126:1241–1253
Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y (2006) The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell 125:85–98
Palevitz BA (1990) Kinetochore behavior during generative cell division in Tradescantia virginiana. Protoplasma 157:120–127
Pinsky BA, Biggins S (2005) The spindle checkpoint: tension versus attachment. Trends Cell Biol 15:486–493
Purcell EM (1977) Life at low Reynolds number. Am J Phys 45:3–11
Reis R, Feijao T, Gouveia S, Pereira AJ, Matos I, Sampaio P, Maiato H, Sunkel CE (2009) Dynein and mast/orbit/CLASP have antagonistic roles in regulating kinetochore-microtubule plus-end dynamics. J Cell Sci 122:2543–2553
Rieder CL (1977) An in-vitro light and electron microscopic study of anaphase chromosome movement in normal and temperature elevated Taricha lung cells. Ph.D. thesis, University of Oregon.
Rieder CL, Schultz A, Cole R, Sluder G (1994) Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol 127:1301–1310
Rieder CL, Cole RW, Khodjakov A, Sluder G (1995) The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 130:941–948
Rogers GC, Rogers SL, Schwimmer TA, Ems-Mcclung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ (2004) Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427:364–370
Sheltzer JM, Blank HM, Pfau SJ, Tange Y, George BM, Humpton TJ, Brito IL, Hiraoka Y, Niwa O, Amon A (2011) Aneuploidy drives genomic instability in yeast. Science 333:1026–1030
Shimamoto Y, Maeda YT, Ishiwata S, Libchaber AJ, Kapoor TM (2011) Insights into the micromechanical properties of the metaphase spindle. Cell 145:1062–1074
Siller KH, Serr M, Steward R, Hays TS, Doe CQ (2005) Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol Biol Cell 16:5127–5140
Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW (2009) Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136:473–484
Straight AF, Marshall WF, Sedat JW, Murray AW (1997) Mitosis in living budding yeast: anaphase A but no metaphase plate. Science 277:574–578
Suzuki A, Hori T, Nishino T, Usukura J, Miyagi A, Morikawa K, Fukagawa T (2011) Spindle microtubules generate tension-dependent changes in the distribution of inner kinetochore proteins. J Cell Biol 193:125–140
Tedeschi A, Ciciarello M, Mangiacasale R, Roscioli E, Rensen WM, Lavia P (2007) RANBP1 localizes a subset of mitotic regulatory factors on spindle microtubules and regulates chromosome segregation in human cells. J Cell Sci 120:3748–3761
Tokai-Nishizumi N, Ohsugi M, Suzuki E, Yamamoto T (2005) The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol Biol Cell 16:5455–5463
Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, McAinsh AD (2009) Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J Cell Biol 184:365–372
Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T (2009) Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol 184:383–390
Uetake Y, Sluder G (2010) Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Curr Biol 20:1666–1671
Walczak CE, Heald R (2008) Mechanisms of mitotic spindle assembly and function. Int Rev Cytol 265:111–158
Wang H, Brust-Mascher I, Cheerambathur D, Scholey JM (2010) Coupling between microtubule sliding, plus-end growth and spindle length revealed by kinesin-8 depletion. Cytoskeleton (Hoboken) 67:715–728
Watanabe K, Hamaguchi MS, Hamaguchi Y (1997) Effects of intracellular pH on the mitotic apparatus and mitotic stage in the sand dollar egg. Cell Motil Cytoskeleton 37:263–270
Waters JC, Mitchison TJ, Rieder CL, Salmon ED (1996) The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell 7:1547–1558
Winey M, Mamay CL, O'Toole ET, Mastronarde DN, Giddings TH Jr, McDonald KL, McIntosh JR (1995) Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J Cell Biol 129:1601–1615
Wuhr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, Mitchison TJ (2008) Evidence for an upper limit to mitotic spindle length. Curr Biol 18:1256–1261
Zhang H, Dawe RK (2011) Mechanisms of plant spindle formation. Chromosome Res 19:335–344
Zhao WM, Coppinger JA, Seki A, Cheng XL, Yates JR 3rd, Fang G (2008) RCS1, a substrate of APC/C, controls the metaphase to anaphase transition. Proc Natl Acad Sci U S A 105:13415–13420
Zheng Y (2010) A membranous spindle matrix orchestrates cell division. Nat Rev Mol Cell Biol 11:529–535
Acknowledgments
The authors would like to thank Sofia Pinho and Eurico Sá for technical help, Conly Rieder, Greg Fitzharris, Jorge Ferreira, Pankaj Dhonukshe, Katja Wassman, Tomohiro Miki, and Gohta Goshima for communication of unpublished data and Jonathon Pines and Patrick Meraldi for comments and critical reading of the manuscript. Work in the laboratory of H.M. is funded by grants PTDC/SAU-GMG/099704/2008 and PTDC/SAU-ONC/112917/2009 from Fundação para a Ciência e a Tecnologia of Portugal (COMPETE-FEDER), the Human Frontier Research Program and the 7th framework program grant PRECISE from the European Research Council.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editors: Rachel O’Neill and Beth Sullivan.
Rights and permissions
About this article
Cite this article
Pereira, A.J., Maiato, H. Maturation of the kinetochore-microtubule interface and the meaning of metaphase. Chromosome Res 20, 563–577 (2012). https://doi.org/10.1007/s10577-012-9298-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-012-9298-8