Abstract
Alzheimer’s disease (AD) is characterized by the increase of hippocampal Ca2+ influx-induced apoptosis and mitochondrial oxidative stress (OS). The OS is a stimulator of TRPM2, although N-(p-amylcinnamoyl)anthranilic acid (ACA), 2-aminoethyl diphenylborinate (2/APB), and glutathione (GSH) are non-specific antagonists of TRPM2. In the present study, we investigated the protective roles of GSH and TRPM2 antagonist treatments on the amyloid β42 peptide (Aβ)-caused oxidative neurotoxicity and apoptosis in the hippocampus of mice with AD model. After the isolation of hippocampal neurons from the newborn mice, they were divided into five incubation groups as follows: control, ACA, Aβ, Aβ+ACA, and Aβ+GSH. The levels of apoptosis, hippocampus death, cytosolic ROS, cytosolic Zn2+, mitochondrial ROS, caspase-3, caspase-9, lipid peroxidation, and cytosolic Ca2+ were increased in the primary hippocampus cultures by treatments of Aβ, although their levels were decreased in the neurons by the treatments of GSH, PARP-1 inhibitors (PJ34 and DPQ), and TRPM2 blockers (ACA and 2/APB). The Aβ-induced decreases of cell viability, cytosolic GSH, reduced GSH, and GSH peroxidase levels were also increased in the groups of Aβ+ACA and Aβ+GSH by the treatments of ACA and GSH. However, the Aβ-caused changes were not observed in the hippocampus of TRPM2-knockout mice. In conclusion, the present data demonstrate that maintaining the activation of TRPM2 is not only important for the quenching OS and neurotoxicity in the hippocampal neurons of mice with experimental AD but also equally critical to the modulation of Aβ-induced apoptosis.
Graphical Abstract
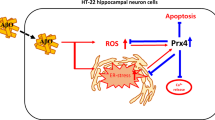
The possible positive effects of GSH and TRPM2 antagonist treatments on the amyloid-beta (Aβ)-induced oxidative toxicity in the hippocampus of mice. The ADP-ribose (ADPR) is produced via the stimulation of PARP-1 in the nucleus of neurons. The NUT9 in the C terminus of TRPM2 channel acts as a key role for the activation of TRPM2. The antagonists of TRPM2 are glutathione (GSH), ACA, and 2/APB in the hippocampus. The Aβ incubation-mediated TRPM2 stimulation increases the concentration of cytosolic-free Ca2+ and Zn2+ in the hippocampus. In turn, the increased concentration causes the increase of mitochondrial membrane potential (ΔΨm), which causes the excessive generations of mitochondria ROS and the decrease of cytosolic GSH and GSH peroxidase (GSH-Px). The ROS production and GSH depletion are two main causes in the neurobiology of Alzheimer’s disease. However, the effect of Aβ was not shown in the hippocampus of TRPM2-knockout mice. The Aβ and TRPM2 stimulation-caused overload Ca2+ entry cause apoptosis and cell death via the activations of caspase-3 (Casp/3) and caspase-9 (Casp/9) in the hippocampus. The actions of Aβ-induced oxidative toxicity were modulated in the primary hippocampus by the incubations of ACA, GSH, 2/APB, and PARP-1 inhibitors (PJ34 and DPQ). (↑) Increase. (↓) Decrease.









Similar content being viewed by others
Data Availability
The incubations and hippocampus isolations in the current study were induced in the Neuroscience Research Center (NÖROBAM), Suleyman Demirel University, Isparta, Turkey. The data were analyzed in BSN Health, Analyses, Innov., Consult., Org., Agricul., Ltd, (Göller Bölgesi Teknokenti, Isparta, Turkey), and the data are available from the Dr. M. Nazıroğlu on reasonable request. All graphics and graphical abstract were prepared in the current study by R. Çınar.
Abbreviations
- ACA:
-
N-(P-amylcinnamoyl)anthranilic acid
- ADPR:
-
ADP-ribose
- AD:
-
Alzheimer’s disease
- BF:
-
Bright field
- Ca2+ :
-
Calcium ion
- Casp/3:
-
Caspase-3
- Casp/9:
-
Caspase-9
- CSM:
-
Confocal laser scanning microscope
- CpX:
-
Cumene hydroperoxide
- cytCa2+ :
-
Cytosolic-free calcium ion
- cytROS:
-
Cytosolic-free reactive oxygen radicals
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- MDA:
-
Malondialdehyde
- mitoPOT:
-
Mitochondrial membrane potential
- mitoROS:
-
Mitochondrial-free reactive oxygen radicals
- TRP:
-
Transient receptor potential
- TRPM2:
-
Transient receptor potential melastatin 2
- 2/APB:
-
2-Aminoethoxydiphenyl borate
- VGCC:
-
Voltage-gated Ca2+ channels
References
Abe K, Misawa M (2003) Amyloid beta protein enhances the clearance of extracellular L-glutamate by cultured rat cortical astrocytes. Neurosci Res 45(1):25–31. https://doi.org/10.1016/s0168-0102(02)00190-6
Abuarab N, Munsey TS, Jiang LH, Li J, Sivaprasadarao A (2017) High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2+-mediated mitochondrial fission. Sci Signal 10(490):eaal4161. https://doi.org/10.1126/scisignal.aal4161
Ai J, Wang H, Chu P, Shopit A, Niu M, Ahmad N et al (2021) The neuroprotective effects of phosphocreatine on Amyloid Beta 25–35-induced differentiated neuronal cell death through inhibition of AKT /GSK-3β /Tau/APP /CDK5 pathways in vivo and vitro. Free Radic Biol Med 162:181–190. https://doi.org/10.1016/j.freeradbiomed.2020.10.003
Akpınar O, Özşimşek A, Güzel M, Nazıroğlu M (2020) Clostridium botulinum neurotoxin A induces apoptosis and mitochondrial oxidative stress via activation of TRPM2 channel signaling pathway in neuroblastoma and glioblastoma tumor cells. J Recept Signal Transduct Res 40(6):620–632. https://doi.org/10.1080/10799893.2020.1781174
Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, Markesbery WR (1998) The expression of key oxidative stress-handling genes in different brain regions in Alzheimer’s disease. J Mol Neurosci 11(2):151–164. https://doi.org/10.1385/JMN:11:2:151
Aliev G, Smith MA, de la Torre JC, Perry G (2004) Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer’s disease. Mitochondrion 4(5–6):649–663. https://doi.org/10.1016/j.mito.2004.07.018
Armağan HH, Nazıroğlu M (2021) Glutathione depletion induces oxidative injury and apoptosis via TRPM2 channel activation in renal collecting duct cells. Chem Biol Interact 334:109306. https://doi.org/10.1016/j.cbi.2020.109306
Balaban H, Nazıroğlu M, Demirci K, Övey İS (2017) The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 54(4):2852–2868. https://doi.org/10.1007/s12035-016-9835-0
Charisis S, Ntanasi E, Yannakoulia M, Anastasiou CA, Kosmidis MH, Dardiotis E et al (2021) Plasma GSH levels and Alzheimer’s disease. A prospective approach.: results from the HELIAD study. Free Radic Biol Med 162:274–282. https://doi.org/10.1016/j.freeradbiomed.2020.10.027
Düzova H, Nazıroğlu M, Çiğ B, Gürbüz P, Akatlı AN (2021) Noopept attenuates diabetes-mediated neuropathic pain and oxidative hippocampal neurotoxicity via inhibition of TRPV1 channel in rats. Mol Neurobiol 58(10):5031–5051. https://doi.org/10.1007/s12035-021-02478-8
Ertilav K (2019) Pregabalin protected cisplatin-induced oxidative neurotoxicity in neuronal cell line. J Cell Neurosci Oxid Stress 11(1):815–824
Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S (2004) TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol 143(1):186–192. https://doi.org/10.1038/sj.bjp.0705914
Fonfria E, Marshall IC, Boyfield I et al (2005) Amyloid beta-peptide(1–42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem 95(3):715–723. https://doi.org/10.1111/j.1471-4159.2005.03396.x
Güzel M, Nazıroğlu M, Akpınar O, Çınar R (2021) Interferon gamma-mediated oxidative stress induces apoptosis, neuroinflammation, zinc ion influx, and TRPM2 channel activation in neuronal cell line: modulator role of curcumin. Inflammation 44(5):1878–1894. https://doi.org/10.1007/s10753-021-01465-4
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658. https://doi.org/10.1111/j.1471-4159.2006.03907.x
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T et al (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9(1):163–173. https://doi.org/10.1016/S1097-2765(01)00438-5
Hong DK, Kho AR, Lee SH, Jeong JH, Kang BS, Kang DH et al (2020) Transient receptor potential melastatin 2 (TRPM2) inhibition by antioxidant, N-acetyl-l-cysteine, reduces global cerebral ischemia-induced neuronal death. Int J Mol Sci 21(17):6026. https://doi.org/10.3390/ijms21176026
Isopi E, Granzotto A, Corona C, Bomba M, Ciavardelli D, Curcio M et al (2015) Pyruvate prevents the development of age-dependent cognitive deficits in a mouse model of Alzheimer’s disease without reducing amyloid and tau pathology. Neurobiol Dis 81:214–224. https://doi.org/10.1016/j.nbd.2014.11.013
Jiang LH, Li X, Syed Mortadza SA, Lovatt M, Yang W (2018) The TRPM2 channel nexus from oxidative damage to Alzheimer’s pathologies: an emerging novel intervention target for age-related dementia. Ageing Res Rev 47:67–79. https://doi.org/10.1016/j.arr.2018.07.002
Joshi DC, Bakowska JC (2011) Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J vis Exp. https://doi.org/10.3791/2704
Keil VC, Funke F, Zeug A, Schild D, Müller M (2011) Ratiometric high-resolution imaging of JC-1 fluorescence reveals the subcellular heterogeneity of astrocytic mitochondria. Pflugers Arch 462:693–708. https://doi.org/10.1007/s00424-011-1012-8
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70. https://doi.org/10.1111/ene.13439
Lee SR (2018) Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev 2018:9156285. https://doi.org/10.1155/2018/9156285
Li X, Jiang LH (2018) Multiple molecular mechanisms form a positive feedback loop driving amyloid β42 peptide-induced neurotoxicity via activation of the TRPM2 channel in hippocampal neurons. Cell Death Dis 9(2):195. https://doi.org/10.1038/s41419-018-0270-1
Lipski J, Park TI, Li D, Lee SC, Trevarton AJ, Chung KK, Freestone PS, Bai JZ (2006) Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res 1077(1):187–199. https://doi.org/10.1016/j.brainres.2006.01.016
Maezawa I, Zou B, Di Lucente J, Cao WS, Pascual C, Weerasekara S, Zhang M, Xie XS, Hua DH, Jin LW (2017) The Anti-Amyloid-β and neuroprotective properties of a novel tricyclic pyrone molecule. J Alzheimers Dis 58(2):559–574. https://doi.org/10.3233/JAD-161175
Marí M, de Gregorio E, de Dios C, Roca-Agujetas V, Cucarull B, Tutusaus A, Morales A, Colell A (2020) Mitochondrial glutathione: recent insights and role in disease. Antioxidants 9(10):909. https://doi.org/10.3390/antiox9100909
Marreiro DD, Cruz KJ, Morais JB, Beserra JB, Severo JS, de Oliveira AR (2017) Zinc and oxidative stress: current mechanisms. Antioxidants 6(2):24. https://doi.org/10.3390/antiox6020024
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6(3):337–350. https://doi.org/10.1111/j.1474-9726.2007.00275.x
Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH (2009) Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci 29(4):1105–1114. https://doi.org/10.1523/JNEUROSCI.4604-08.2009
Mei ZZ, Mao HJ, Jiang LH (2006) Conserved cysteine residues in the pore region are obligatory for human TRPM2 channel function. Am J Physiol Cell Physiol 291(5):C1022-1028. https://doi.org/10.1152/ajpcell.00606.2005
Meng X, Fu M, Wang S, Chen W, Wang J, Zhang N (2021) Naringin ameliorates memory deficits and exerts neuroprotective effects in a mouse model of Alzheimer’s disease by regulating multiple metabolic pathways. Mol Med Rep 23(5):332. https://doi.org/10.3892/mmr.2021.11971
Misrani A, Tabassum S, Yang L (2021) Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front Aging Neurosci 13:617588. https://doi.org/10.3389/fnagi.2021.617588
Mortadza SS, Sim JA, Stacey M, Jiang LH (2017) Signalling mechanisms mediating Zn2+-induced TRPM2 channel activation and cell death in microglial cells. Sci Rep 7:45032. https://doi.org/10.1038/srep45032
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32(11):1990–2001
Nazıroğlu M, Lückhoff A (2008) Effects of antioxidants on calcium influx through TRPM2 channels in transfected cells activated by hydrogen peroxide. J Neurol Sci 270(1–2):152–158. https://doi.org/10.1016/j.jns.2008.03.003
Nazıroğlu M, Özgül C, Çiğ B, Doğan S, Uğuz AC (2011) Glutathione modulates Ca(2+) influx and oxidative toxicity through TRPM2 channel in rat dorsal root ganglion neurons. J Membr Biol 242(3):109–118. https://doi.org/10.1007/s00232-011-9382-6
Nazıroğlu M, Muhamad S, Pecze L (2017) Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: focus on selenium nanoparticles. Expert Rev Clin Pharmacol 10(7):773–782. https://doi.org/10.1080/17512433.2017.1324781
Övey İS, Naziroğlu M (2015) Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience 284:225–233. https://doi.org/10.1016/j.neuroscience.2014.09.078
Övey İS, Nazıroğlu M (2021) Effects of homocysteine and memantine on oxidative stress related TRP cation channels in in-vitro model of Alzheimer’s disease. J Recept Signal Transduct Res 41(3):273–283. https://doi.org/10.1080/10799893.2020.1806321
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon JS (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41:101947. https://doi.org/10.1016/j.redox.2021.101947
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C et al (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411(6837):595–599
Rigotto G, Zentilin L, Pozzan T, Basso E (2021) Effects of mild excitotoxic stimulus on mitochondria Ca2+ handling in hippocampal cultures of a mouse model of Alzheimer’s disease. Cells 10(8):2046. https://doi.org/10.3390/cells10082046
Seuma M, Faure AJ, Badia M, Lehner B, Bolognesi B (2021) The genetic landscape for amyloid beta fibril nucleation accurately discriminates familial Alzheimer’s disease mutations. Elife 10:e63364. https://doi.org/10.7554/eLife.63364
Sharma S, Ebadi M (2014) Significance of metallothioneins in aging brain. Neurochem Int 65:40–48. https://doi.org/10.1016/j.neuint.2013.12.009
Soto-Mercado V, Mendivil-Perez M, Velez-Pardo C, Lopera F, Jimenez-Del-Rio M (2020) Cholinergic-like neurons carrying PSEN1 E280A mutation from familial Alzheimer’s disease reveal intraneuronal sAPPβ fragments accumulation, hyperphosphorylation of TAU, oxidative stress, apoptosis and Ca2+ dysregulation: therapeutic implications. PLoS ONE 15(5):e0221669. https://doi.org/10.1371/journal.pone.0221669
Sultan FA (2013) Dissection of different areas from mouse hippocampus. Bio Protoc 3(21):e955. https://doi.org/10.21769/bioprotoc.955
Thapak P, Vaidya B, Joshi HC, Singh JN, Sharma SS (2020) Therapeutic potential of pharmacological agents targeting TRP channels in CNS disorders. Pharmacol Res 159:105026. https://doi.org/10.1016/j.phrs.2020.105026
Tsai CW, Tsai CF, Lin KH, Chen WJ, Lin MS, Hsieh CC, Lin CC (2020) An investigation of the correlation between the S-glutathionylated GAPDH levels in blood and Alzheimer’s disease progression. PLoS ONE 15(5):e0233289. https://doi.org/10.1371/journal.pone.0233289
Walia V, Kaushik D, Mittal V, Kumar K, Verma R, Parashar J et al (2022) Delineation of neuroprotective effects and possible benefits of antioxidants therapy for the treatment of Alzheimer’s diseases by targeting mitochondrial-derived reactive oxygen species: bench to bedside. Mol Neurobiol 59(1):657–680. https://doi.org/10.1007/s12035-021-02617-1
Wang X, Zheng W (2019) Ca2+ homeostasis dysregulation in Alzheimer’s disease: a focus on plasma membrane and cell organelles. FASEB J 33(6):6697–6712. https://doi.org/10.1096/fj.201801751R
World Alzheimer Report 2019 Attitudes to Dementia (2019). https://www.alzint.org/research/%20WorldAlzheimerReport2019.pdf. Accessed 15 May 2022
Yang W, Manna PT, Zou J, Luo J, Beech DJ, Sivaprasadarao A, Jiang LH (2011) Zinc inactivates melastatin transient receptor potential 2 channels via the outer pore. J Biol Chem 286(27):23789–23798. https://doi.org/10.1074/jbc.M111.247478
Ye M, Yang W, Ainscough JF, Hu XP et al (2014) TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis 5(11):e1541. https://doi.org/10.1038/cddis.2014.494
Yildizhan K, Çınar R, Naziroğlu M (2022) The involvement of TRPM2 on the MPP+-induced oxidative neurotoxicity and apoptosis in hippocampal neurons from neonatal mice: protective role of resveratrol. Neurol Res. https://doi.org/10.1080/01616412.2022.2027644
Yu P, Wang Q, Zhang LH, Lee HC, Zhang L, Yue J (2012) A cell permeable NPE caged ADP-ribose for studying TRPM2. PLoS ONE 7(12):e51028. https://doi.org/10.1371/journal.pone.0051028
Acknowledgements
Data of the present study were summarized from PhD Thesis of R. Çınar (Supervisor: Professor M. Nazıroğlu).
Funding
Scientific Research Project Unit (BAP) of Suleyman Demirel University (Isparta, Turkey) financially supported the project (Project No: TDK-2020-7454: Project Owner: Professor M. Nazıroğlu). Research fellowship of R. Çınar was paid in the project by the 100/2000 program of Turkish Higher Education Council (Ankara, Turkey).
Author information
Authors and Affiliations
Contributions
MN prepared the manuscript and designed the project. RÇ performed hippocampal neuron isolation, plate reader, laser confocal microscope, and spectrofluorometer analyses. The final manuscript submission was approved by the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
There is no human or human sample in the current study. The present study was conducted according to the approval of the Experimental Animal Ethics Committee of Burdur Mehmet Akif University (Date: 18.12.2019. Approve number: 594). The authors declare that there is no ethical conflict to disclose.
Consent to Participate
Dr. Nazıroğlu prepared the manuscript and designed the project. Mr. R. Çınar performed hippocampal neuron isolation, plate reader, laser confocal microscope, and spectrofluorometer analyses. The final manuscript submission was approved by the authors.
Consent for Publication
All authors approved the final manuscript as submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Çınar, R., Nazıroğlu, M. TRPM2 Channel Inhibition Attenuates Amyloid β42-Induced Apoptosis and Oxidative Stress in the Hippocampus of Mice. Cell Mol Neurobiol 43, 1335–1353 (2023). https://doi.org/10.1007/s10571-022-01253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01253-0