Abstract
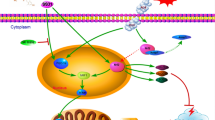
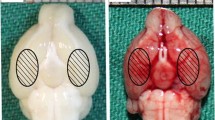
Mitochondrial dysfunction is considered a crucial therapeutic target for early brain injury following subarachnoid hemorrhage (SAH). Emerging evidence indicates that docosahexaenoic acid (DHA), an essential omega-3 fatty acid, protects mitochondria in various chronic diseases. This study aimed to investigate the neuroprotective effects of DHA on mitochondrial dynamic dysfunction after EBI using in vivo and in vitro approaches. For in vivo experiments, the rat endovascular perforation SAH model was performed, whereby DHA was administered intravenously 1 h after induction of SAH. Primary cultured neurons treated with oxyhemoglobin (OxyHb) for 24 h were used to mimic SAH in vitro. Our results demonstrated that DHA improved neurological deficits and reduced brain edema in rats with SAH, and attenuated OxyHb-induced neuronal death in primary cultured cells. DHA reduced the amount of reactive oxygen species-positive cells and improved cell viability when compared to the SAH + vehicle group in vitro. DHA attenuated malondialdehyde levels and superoxide dismutase stress, increased Bcl2 and Bcl-xl, and decreased Bax and cleaved caspase-3 in vivo. Additionally, DHA ameliorated mitochondrial dysfunction, upregulated the mitochondrial fusion-related protein Optic Atrophy 1, and downregulated the mitochondrial fission-related protein Dynamin-Related-Protein 1 (Drp1) and Serine 616 phosphorylated Drp1 after SAH both in vitro and in vivo. Taken together, our current study demonstrates that DHA might prevent oxidative stress-based apoptosis after SAH. The characterization of the underlying molecular mechanisms may further improve mitochondrial dynamics-related signaling pathways.






Similar content being viewed by others
References
Afshordel S, Hagl S, Werner D, Röhner N, Kögel D, Bazan NG, Eckert GP (2015) Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging—impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot Essent Fatty Acids 92:23–31
Archer SL (2013) Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med 369:2236–2251
Baburamani AA, Hurling C, Stolp H, Sobotka K, Gressens P, Hagberg H, Thornton C (2015) Mitochondrial optic atrophy (OPA) 1 processing is altered in response to neonatal hypoxic-ischemic brain injury. Int J Mol Sci 16:22509–22526
Balog J, Mehta SL, Vemuganti R (2016) Mitochondrial fission and fusion in secondary brain damage after CNS insults. J Cereb Blood Flow Metab 36:2022–2033
Bazan NG (2005) Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol 15:159–166
Bazan NG, Calandria JM, Gordon WC (2013) Docosahexaenoic acid and its derivative neuroprotectin D1 display neuroprotective properties in the retina, brain and central nervous system. Nestle Nutr Inst Workshop Ser 77:121–131
Bertholet AM et al (2016) Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis 90:3–19
Burnside SW, Hardingham GE (2017) Transcriptional regulators of redox balance and other homeostatic processes with the potential to alter neurodegenerative disease trajectory. Biochem Soc Trans 45:1295–1303
Cai J, Cao S, Chen J, Yan F, Chen G, Dai Y (2015) Progesterone alleviates acute brain injury via reducing apoptosis and oxidative stress in a rat experimental subarachnoid hemorrhage model. Neurosci Lett 600:238–243
Chen S et al (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91
Chen S, Wu H, Tang J, Zhang J, Zhang JH (2015) Neurovascular events after subarachnoid hemorrhage: focusing on subcellular organelles. Acta Neurochir Suppl 120:39–46
Connolly ES Jr et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43:1711–1737
Cui Y et al (2016) Hydrogen sulfide ameliorates early brain injury following subarachnoid hemorrhage in rats. Mol Neurobiol 53:3646–3657
Cui C, Song S, Cui J, Feng Y, Gao J, Jiang P (2017) Vitamin D receptor activation influences NADPH oxidase (NOX2) activity and protects against neurological deficits and apoptosis in a rat model of traumatic brain injury. Oxid Med Cell Longev. https://doi.org/10.1155/2017/9245702
Dai J et al (2017) Changes in mitochondrial ultrastructure in SH-SY5Y cells during apoptosis induced by hemin. Neuroreport 28:551–554
Dai Y, Zhang H, Zhang J, Yan M (2018) Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem Biol Interact 284:32–40
Echigo R et al (2012) Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J Transl Med 10:80
Fan LF et al (2017) Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol Med 112:336–349
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4:432–446
Gollihue JL, Patel SP, Rabchevsky AG (2018) Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma. Neural Regen Res 13:194–197
Golpich M, Amini E, Mohamed Z, Azman Ali R, Ibrahim NM, Ahmadiani A (2017) Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther 23:5–22
Guo M et al (2018) Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci 11:86
Huang J et al (2001) Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci USA 98:11720–11724
Huang L, Sherchan P, Wang Y, Reis C, Applegate RL 2nd, Tang J, Zhang JH (2015) Phosphoinositide 3-kinase gamma contributes to neuroinflammation in a rat model of surgical brain injury. J Neurosci 35:10390–10401
Iannielli A, Bido S, Folladori L, Segnali A, Cancellieri C, Maresca A, Massimino L et al (2018) Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in Parkinson’s disease models. Cell Rep 22:2066–2079
Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G (2012) Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience 213:144–153
Lee H, Smith SB, Yoon Y (2017) The short variant of the mitochondrial dynamin OPA1 maintains mitochondrial energetics and cristae structure. J Biol Chem 292:7115–7130
Li Q et al (2016) Hemoglobin induced NO/cGMP suppression deteriorate microcirculation via pericyte phenotype transformation after subarachnoid hemorrhage in rats. Sci Rep 6:22070
Liu W, Chen X, Zhang Y (2016) Effects of microRNA-21 and microRNA-24 inhibitors on neuronal apoptosis in ischemic stroke. Am J Transl Res 8:3179–3187
MacVicar TD, Lane JD (2014) Impaired OMA1-dependent cleavage of OPA1 and reduced DRP1 fission activity combine to prevent mitophagy in cells that are dependent on oxidative phosphorylation. J Cell Sci 127:2313–2325
Mayurasakorn K et al (2016) DHA but not EPA emulsions preserve neurological and mitochondrial function after brain hypoxia-ischemia in neonatal mice. PLoS ONE 11:e0160870
Meguro T, Chen B, Lancon J, Zhang JH (2001) Oxyhemoglobin induces caspase-mediated cell death in cerebral endothelial cells. J Neurochem 77:1128–1135
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278:7743–7746
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922
Prasansuklab A, Meemon K, Sobhon P, Tencomnao T (2017) Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complement Altern Med 17:551
Prentice H, Modi JP, Wu JY (2015) Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev 2015:964518
Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67:103–118
Regmi SG, Rolland SG, Conradt B (2014) Age-dependent changes in mitochondiral morphology and volume are not predictors of lifespan. Aging 6:118–130
Rego AC, Oliveira CR (2003) Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28:1563–1574
Rodriguez-Carrizalez AD et al (2014) Oxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathy. J Diabetes 6:167–175
Sharp WW et al (2014) Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28:316–326
Shi Z et al (2016) Enriched endogenous omega-3 polyunsaturated fatty acids protect cortical neurons from experimental ischemic injury. Mol Neurobiol 53:6482–6488
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167:327–334
Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH (2010) Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol 68:650–660
Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7
Wu P et al (2017) Mdivi-1 alleviates early brain injury after experimental subarachnoid hemorrhage in rats, possibly via inhibition of Drp1-activated mitochondrial fission and oxidative stress. Neurochem Res 42:1449–1458
Yin J et al (2016) Inhibitory effects of omega-3 fatty acids on early brain injury after subarachnoid hemorrhage in rats: possible involvement of G protein-coupled receptor 120/β-arrestin2/TGF-β activated kinase-1 binding protein-1 signaling pathway. Int J Biochem Cell Biol 75:11–22
Zhang L et al (2014) Exercise pretreatment promotes mitochondrial dynamic protein OPA1 expression after cerebral ischemia in rats. Int J Mol Sci 15:4453–4463
Acknowledgements
This study was funded by the Fundamental Research Funds for the Provincial Universities (2017LCZX30).
Author information
Authors and Affiliations
Contributions
All authors listed contributed immensely to this study. TZ and PW performed the experiments, wrote the paper. YL, YL, and BL helped to perform the animal experiments. CW, LW, GZ, JD, SZ analyzed the data. HS, JHZ, and S X, as experts in neurology, provided technical supports and designed the research. Cesar Reis has proofread this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, T., Wu, P., Zhang, J.H. et al. Docosahexaenoic Acid Alleviates Oxidative Stress-Based Apoptosis Via Improving Mitochondrial Dynamics in Early Brain Injury After Subarachnoid Hemorrhage. Cell Mol Neurobiol 38, 1413–1423 (2018). https://doi.org/10.1007/s10571-018-0608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0608-3