Abstract
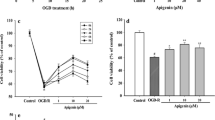
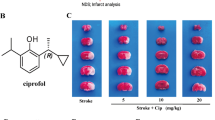
Oxidative stress is a great challenge to neurons following cerebral ischemia. PGC-1α has been shown to act as a potent modulator of oxidative metabolism. In this study, the effects of ZLN005, a small molecule that activate PGC-1α, against oxygen–glucose deprivation (OGD)- or ischemia-induced neuronal injury in vitro and in vivo were investigated. Transient middle cerebral artery occlusion (tMCAO) was performed in rats and ZLN005 was administered intravenously at 2 h, 4 h, or 6 h after ischemia onset. Infarct volume and neurological deficit score were detected to evaluate the neuroprotective effects of ZLN005. Well-differentiated PC12 cells, which were subjected to OGD for 2 h followed by reoxygenation for 22 h, were used as an in vitro ischemic model. Changes in expression of PGC-1α, its related genes, and antioxidant genes were determined by real-time quantitative PCR. The results showed that ZLN005 reduced cerebral infarct volume and improved the neurological deficit in rat with tMCAO, and significantly protected OGD-induced neuronal injury in PC12 cells. Furthermore, ZLN005 enhanced expression of PGC-1α in PC12 cells and in the ipsilateral hemisphere of rats with tMCAO. Additionally, ZLN005 increased antioxidant genes, including SOD1 and HO-1, and significantly prevented the ischemia-induced decrease in SOD activity. Taking together, the PGC-1α activator ZLN005 exhibits neuroprotective effects under ischemic conditions and molecular mechanisms possibly involve activation of PGC-1α signaling pathway and cellular antioxidant systems.






Similar content being viewed by others
References
Bian M et al (2016) Celastrol protects mouse retinas from bright light-induced degeneration through inhibition of oxidative stress and inflammation. J Neuroinf 13:50. https://doi.org/10.1186/s12974-016-0516-8
Chang R et al (2016) Protective effects of aloin on oxygen and glucose deprivation-induced injury in PC12 cells. Brain Res Bull 121:75–83. https://doi.org/10.1016/j.brainresbull.2016.01.001
Chang S et al (2017) The natural product 4,10-aromadendranediol induces neuritogenesis in neuronal cells in vitro through activation of the ERK pathway. Acta Pharmacol Sin 38:29–40. https://doi.org/10.1038/aps.2016.115
Chen SD et al (2010) Activation of calcium/calmodulin-dependent protein kinase IV and peroxisome proliferator-activated receptor γ coactivator-1α signaling pathway protects against neuronal injury and promotes mitochondrial biogenesis in the hippocampal CA1 subfield after transient global ischemia. J Neurosci Res 88:3144–3154. https://doi.org/10.1002/jnr.22469
Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC (2011) Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 12:7199–7215. https://doi.org/10.3390/ijms12107199
Chen T et al (2014) Nafamostat mesilate attenuates neuronal damage in a rat model of transient focal cerebral ischemia through thrombin inhibition. Sci Rep 4:5531. https://doi.org/10.1038/srep05531
di Penta A et al (2013) Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS ONE 8:e54722. https://doi.org/10.1371/journal.pone.0054722
Ding Y, Chen M, Wang M, Li Y, Wen A (2015) Posttreatment with 11-Keto-β-Boswellic acid ameliorates cerebral ischemia-reperfusion injury: nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol 52:1430–1439. https://doi.org/10.1007/s12035-014-8929-9
Gao Y et al (2015) Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: potential roles of Akt activation and HO-1 induction. Toxicol Appl Pharmacol 289:142–154. https://doi.org/10.1016/j.taap.2015.10.001
Garcia G et al (2017) Bioaccessible (poly)phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem 215:274–283. https://doi.org/10.1016/j.foodchem.2016.07.128
García-Quintans N et al (2016) Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 19:217–228. https://doi.org/10.1007/s10456-016-9502-0
Gu WW et al (2016) 2-(3′,5′-Dimethoxybenzylidene) cyclopentanone, a novel synthetic small-molecule compound, provides neuroprotective effects against ischemic stroke. Neuroscience 316:26–40. https://doi.org/10.1016/j.neuroscience.2015.11.052
Ho YH, Lin YT, Wu CW, Chao YM, Chang AY, Chan JY (2015) Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J Biomed Sci 22:46. https://doi.org/10.1186/s12929-015-0157-8
Li L et al (2015) Chinese herbal medicine formula tao hong si wu decoction protects against cerebral ischemia-reperfusion injury via PI3 K/Akt and the Nrf2 signaling pathway. J Nat Med 69:76–85. https://doi.org/10.1007/s11418-014-0865-5
Li L et al (2016a) Sestrin2 silencing exacerbates cerebral ischemia/reperfusion injury by decreasing mitochondrial biogenesis through the AMPK/PGC-1α pathway in rats. Sci Rep 6:30272. https://doi.org/10.1038/srep30272
Li W et al (2016b) ZLN005 protects cardiomyocytes against high glucose-induced cytotoxicity by promoting SIRT1 expression and autophagy. Exp Cell Res 345:25–36. https://doi.org/10.1016/j.yexcr.2016.05.012
Liu P et al (2015) MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 46:513–519. https://doi.org/10.1161/STROKEAHA.114.007482
Liu X, Zhu X, Chen M, Ge Q, Shen Y, Pan S (2016) Resveratrol protects PC12 cells against OGD/R-induced apoptosis via the mitochondrial-mediated signaling pathway. Acta Biochim Biophys Sin (Shanghai) 48:342–353. https://doi.org/10.1093/abbs/gmw011
Liu SG, Wang YM, Zhang YJ, He XJ, Ma T, Song W, Zhang YM (2017) ZL006 protects spinal cord neurons against ischemia-induced oxidative stress through AMPK-PGC-1α-Sirt3 pathway. Neurochem Int 108:230–237. https://doi.org/10.1016/j.neuint.2017.04.005
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Ma X, Xie Y, Chen Y, Han B, Li J, Qi S (2016) Post-ischemia mdivi-1 treatment protects against ischemia/reperfusion-induced brain injury in a rat model. Neurosci Lett 632:23–32. https://doi.org/10.1016/j.neulet.2016.08.026
Mäkelä J, Tselykh TV, Kukkonen JP, Eriksson O, Korhonen LT, Lindholm D (2016) Peroxisome proliferator-activated receptor-γ (PPARγ) agonist is neuroprotective and stimulates PGC-1α expression and CREB phosphorylation in human dopaminergic neurons. Neuropharmacology 102:266–275. https://doi.org/10.1016/j.neuropharm.2015.11.020
Mossakowski AA et al (2015) Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol 130:799–814. https://doi.org/10.1007/s00401-015-1497-x
Mudò G et al (2012) Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci 69:1153–1165. https://doi.org/10.1007/s00018-011-0850-z
O’Hare Doig RL, Bartlett CA, Maghzal GJ, Lam M, Archer M, Stocker R, Fitzgerald M (2014) Reactive species and oxidative stress in optic nerve vulnerable to secondary degeneration. Exp Neurol 261:136–146. https://doi.org/10.1016/j.expneurol.2014.06.007
Pang T et al (2016) A novel GSK-3β inhibitor YQ138 prevents neuronal injury induced by glutamate and brain ischemia through activation of the Nrf2 signaling pathway. Acta Pharmacol Sin 37:741–752. https://doi.org/10.1038/aps.2016.3
Park JH, Park YS, Lee JB, Park KH, Paik MK, Jeong M, Koh HC (2016) Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. J Appl Toxicol 36:10–23. https://doi.org/10.1002/jat.3136
Peng H et al (2015) Lack of PGC-1α exacerbates high glucose-induced apoptosis in human umbilical vein endothelial cells through activation of VADC1. Int J Clin Exp Pathol 8:4639–4650
Qi D et al (2014) HO-1 attenuates hippocampal neurons injury via the activation of BDNF-TrkB-PI3 K/Akt signaling pathway in stroke. Brain Res 1577:69–76. https://doi.org/10.1016/j.brainres.2014.06.031
Qiao H et al (2012) Protective effect of luteolin in experimental ischemic stroke: upregulated SOD1, CAT, Bcl-2 and claudin-5, down-regulated MDA and Bax expression. Neurochem Res 37:2014–2024. https://doi.org/10.1007/s11064-012-0822-1
Ritzel RM et al (2016) Early retinal inflammatory biomarkers in the middle cerebral artery occlusion model of ischemic stroke. Mol Vis 22:575–588
Sharma DR, Sunkaria A, Wani WY, Sharma RK, Kandimalla RJ, Bal A, Gill KD (2013) Aluminium induced oxidative stress results in decreased mitochondrial biogenesis via modulation of PGC-1α expression. Toxicol Appl Pharmacol 273:365–380. https://doi.org/10.1016/j.taap.2013.09.012
Shirley R, Ord EN, Work LM (2014) Oxidative stress and the use of antioxidants in stroke. Antioxidants (Basel) 3:472–501. https://doi.org/10.3390/antiox3030472
Shulyakova N, Sidorova-Darmos E, Fong J, Zhang G, Mills LR, Eubanks JH (2014) Over-expression of the Sirt3 sirtuin Protects neuronally differentiated PC12 Cells from degeneration induced by oxidative stress and trophic withdrawal. Brain Res 1587:40–53. https://doi.org/10.1016/j.brainres.2014.08.066
Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L (2016) PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat 125:8–18. https://doi.org/10.1016/j.prostaglandins.2016.07.004
St-Pierre J et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Szalardy L, Molnar M, Torok R, Zadori D, Kovacs GG, Vecsei L, Klivenyi P (2016) Lack of age-related clinical progression in PGC-1α-deficient mice – implications for mitochondrial encephalopathies. Behav Brain Res 313:272–281. https://doi.org/10.1016/j.bbr.2016.07.021
Wang LQ et al (2014) Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci Ther 20:317–326. https://doi.org/10.1111/cns.12216
Wang Y et al (2017a) A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7003
Wang Y et al (2017b) Balasubramide derivative 3C modulates microglia activation via CaMKKβ-dependent AMPK/PGC-1α pathway in neuroinflammatory conditions. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2017.08.006
Won YW, Lee M, Kim HA, Bull DA, Kim SW (2013) Hypoxia-inducible plasmid expressing both miSHP-1 and HO-1 for the treatment of ischemic disease. J Control Release 165:22–28. https://doi.org/10.1016/j.jconrel.2012.10.014
Wu Y, Shang Y, Sun SG, Liu RG, Yang WQ (2007) Protective effect of erythropoietin against 1-methyl-4-phenylpyridinium-induced neurodegenaration in PC12 cells. Neurosci Bull 23:156–164
Wu KL, Wu CW, Chao YM, Hung CY, Chan JY (2016) Impaired Nrf2 regulation of mitochondrial biogenesis in rostral ventrolateral medulla on hypertension induced by systemic inflammation. Free Radic Biol Med 97:58–74. https://doi.org/10.1016/j.freeradbiomed.2016.05.012
Xiao W, Goswami PC (2015) Down-regulation of peroxisome proliferator activated receptor gamma coactivator 1α induces oxidative stress and toxicity of 1-(4-Chlorophenyl)-benzo-2,5-quinone in HaCaT human keratinocytes. Toxicol In Vitro 29:1332–1338. https://doi.org/10.1016/j.tiv.2015.05.009
Xu Y et al (2015) Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain Behav Immun 50:298–313. https://doi.org/10.1016/j.bbi.2015.07.015
Xue F et al (2016) Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res 309:1–8. https://doi.org/10.1016/j.bbr.2016.04.045
Yang F et al (2014) NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab 34:660–667. https://doi.org/10.1038/jcbfm.2013.242
Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J (2008) Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39:3057–3063. https://doi.org/10.1161/STROKEAHA.108.520114
Yu S, Cheng Q, Li L, Liu M, Yang Y, Ding F (2014) 2-(4-Methoxyphenyl)ethyl-2-acetamido-2-deoxy-beta-d-pyranoside confers neuroprotection in cell and animal models of ischemic stroke through calpain1/PKA/CREB-mediated induction of neuronal glucose transporter 3. Toxicol Appl Pharmacol 277:259–269. https://doi.org/10.1016/j.taap.2014.03.025
Zhang LN et al (2013) Novel small-molecule PGC-1α transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes 62:1297–1307. https://doi.org/10.2337/db12-0703
Zhang J et al (2014) Bicyclol upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res Bull 100:38–43. https://doi.org/10.1016/j.brainresbull.2013.11.001
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21402241), the Natural Science Foundation of Jiangsu Province (BK20160032), the Six Talent Peaks Project of Jiangsu Province (T.P.), and the Program for Jiangsu Province “Shuang Chuang” Team.
Author information
Authors and Affiliations
Contributions
All authors listed contributed immensely to this study. YX and JAK performed the experiments and wrote the paper. WR, YW, SZ, and XS performed the animal experiments and analyzed the data. TP, JL, and LZ, as experts in molecular pharmacology, provided technical supports and designed the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Xu, Y., Kabba, J.A., Ruan, W. et al. The PGC-1α Activator ZLN005 Ameliorates Ischemia-Induced Neuronal Injury In Vitro and In Vivo. Cell Mol Neurobiol 38, 929–939 (2018). https://doi.org/10.1007/s10571-017-0567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0567-0