Abstract
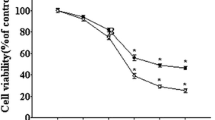
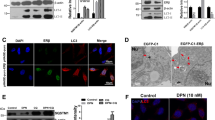
Senile plaque accumulation and neurofibrillary tangles are primary characteristics of Alzheimer’s disease. We aimed to assess the protective functions of naringenin against β-amyloid protein fragment 25-35 (Aβ25-35)-caused nerve damage in differentiated PC12 cells, and study the potential mechanisms. We evaluated cell viability and apoptosis using the 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) test and flow cytometry, respectively. Moreover, we measured protein kinase B (Akt), glycogen synthase kinase-3β (GSK-3β), and caspase-3 activity via western blotting and RT-PCR. We found that naringenin protected cell against Aβ25-35-caused nerve damage by increasing cell viability, promoting Akt and GSK3β activation, and inhibiting cell apoptosis and caspase-3 activity. However, treatment with the estrogen receptor (ER) antagonist ICI182, 780 or phosphatidylinositol-3-kinase (PI3K) inhibitor LY294002 suppressed the effects of naringenin. Our results suggested that naringenin could effectively suppress Aβ25-35-caused nerve damage in PC12 cells by regulating the ER and PI3K/Akt pathways.






Similar content being viewed by others
References
Amer Dena AM, Georg K, Nicole M et al (2010) Activation of transgenic estrogen receptor-beta by selected phytoestrogens in a stably transduced rat serotonergic cell line. J Steroid Biochem Mol Biol 120:208–217
Astrid GZ, Ross S, Wu ZX et al (2005) Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BioMed Central 6:54
Cao GS, Li SX, Wang Y et al (2016) A combination of four effective components derived from Sheng-mai san attenuates hydrogen peroxide-induced injury in PC12 cells through inhibiting Akt and MAPK signaling pathways. Chin J Nat Med 14:0508–0517
Chen WF, Zhou LP, Chen L et al (2013) Involvement of IGF-I receptor and estrogen receptor pathways in the protective effects of ginsenoside Rg1 against Aβ25-35-induced toxicity in PC12 cells. Neurochem Int 62:1065–1071
Dong YL, Yang N, Liu YY et al (2011) The neuroprotective effects of phytoestrogen α-zearalanol on β-amyloid-induced toxicity in differentiated PC-12 cells. Eur J Pharmacol 670:392–398
Feng GF, Wang WX, Qian YH et al (2013) Anti-Aβ antibodies induced by Aβ-HBc virus-like particles prevent Aβ aggregation and protect PC12 cells against toxicity of Aβ1-40. J Neurosci Methods 218:48–54
Ghofrani S, Joghataei MT, Mohseni S et al (2015) Naringenin improves learning and memory in an Alzheimer’s disease rat model: insights into the underlying mechanisms. Eur J Pharmacol 764:195–201
Heo HJ, Kim DO, Shin SC et al (2004) Effect of antioxidant flavanone, naringenin, from citrus junos on neuroprotection. J Agric Food Chem 52:1520–1525
Hou XQ, Yan R, Yang C et al (2014) A novel assay for high-throughput screening of anti-Alzheimer’s disease drugs to determine their efficacy by real-time monitoring of changes in PC12 cell proliferation. Int J Mol Med 33:543–549
Kannappan S, Palanisamy N, Anuradha CV (2010) Suppression of hepatic oxidative events and regulation of eNOS expression in the liver by naringenin in fructose-administered rats. Eur J Pharmacol 645:177–184
Lee JH, Jiang Y, Han DH et al (2014) Targeting estrogen receptors for the treatment of alzheimer’s disease. Mol Neurobiol 49:39–49
Meng XB, Wang M, Sun GB et al (2014) Attenuation of Aβ25–35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol Appl Pharmacol 279:63–75
Park HJ, Choi YJ, Lee JH et al (2017) Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem Toxicol 99:1–8
Shi C, Zhu XM, Wang JS et al (2014) Tanshinone IIA promotes non-amyloidogenic processing of amyloid precursor protein in platelets via estrogen receptor signaling to phosphatidylinositol 3-kinase/Akt. Biomed Rep 2:500–504
Sun ZK, Yang HQ, Wang ZQ et al (2012) Erythropoietin prevents PC12 cells from beta-amyloid-induced apoptosis via PI3K/Akt pathway. Transl Neurodegener 1:7
Wang Z, ZHANG X, Wang H et al (2007) Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 145:911–922
Xian YF, Lin ZX, Ip SP et al (2013) Comparison the neuropreotective effect of Cortex Phellodendri chinensis and Cortex Phellodendri amurensis against beta-amyloid-induced neurotoxicity in PC12 cells. Phytomedicine 20:187–193
Xiao ZM, Huang G, Wu J et al (2013) The neuroprotective effects of ipriflavone against H2O2 and amyloid beta induced toxicity in human neuroblastoma SH-SY5Y cells. Eur J Pharmacol 721:286–293
Xing GH, Dong MX, Li XM et al (2011) Neuroprotective effects of puerarin against beta-amyloid-induced neurotoxicity in PC12 cells via a PI3K-dependent signaling pathway. Brain Res Bull 85:212–218
Yang WQ, Ma J, Yu HR et al (2013) Improvement of naringenin on cognition of model rats with Alzheimer’s diseases and its mechanism. Chin Tradit Herb Drugs 44:715–720
Zhao L, Chen Q, Brinton RD et al (2002) Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp BiolMed 227:509–519
Zhao LQ, Mao ZS, Chen SH et al (2013) Early intervention with an estrogen receptor β-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of alzheimer’s disease. J Alzheimer’s Dis 37:403–419
Zou Y, Hong B, Fan L et al (2013) Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3β/Nrf2 signaling pathway. Free Radic Res 47(1):55–63
Acknowledgements
This work was supported by National Natural Science Foundation of China (General Program, Grant No. 81673581), Harbin science and technology innovation talent research special funds (rqyxj012 outstanding youth talent, 2016), and Heilongjiang university of Chinese medicine scientific research fund (2015 xy04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, N., Hu, Z., Zhang, Z. et al. Protective Role Of Naringenin Against Aβ25-35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell Mol Neurobiol 38, 549–557 (2018). https://doi.org/10.1007/s10571-017-0519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0519-8