Abstract
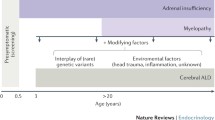
X-linked adrenoleukodystrophy (X-ALD) is the most frequent peroxisomal disorder that is characterized by progressive demyelination of the white matter, adrenal insufficiency, and accumulation of very long-chain fatty acids in body fluid and tissues. This disorder is clinically heterogeneous with seven different phenotypes in male patients and five phenotypes in female carriers. An ultimate treatment for X-ALD is not available. Depending on the rate of the disease progression and the degree of an individual handicap, special needs and challenges vary greatly. The exact mechanisms underlying the pathophysiology of this multifactorial neurodegenerative disorder remains obscure. Previous studies has been related oxidative stress with the pathogenesis of several disease that affecting the central nervous system, such as neurodegenerative disease, epilepsy, multiple sclerosis, Alzheimer, and Parkinson diseases. In addition, oxidative damage has been observed in various in vivo and in vitro studies with inborn errors of metabolism, including X-ALD. In this context, this review is focused on oxidative stress in X-ALD, with emphasis on studies using biological samples from patients affected by this disease.

Similar content being viewed by others
Abbreviations
- AASA:
-
Aminoadipic semialdehyde
- ABCD1:
-
ATP-binding cassette (ABC) transporter subfamily D member 1
- ABAP:
-
2,2′-Azo-bis-(2-aminidinopropane)
- AMN:
-
Adrenomyeloneuropathy
- ATP:
-
Adenosine triphosfate
- BMT:
-
Bone marrow transplant
- C22:0:
-
Docosanoic acid
- C24:0:
-
Tetracosanoic acid
- C26:0:
-
Hexacosanoic acid
- Ca2+ :
-
Calcium
- CAT:
-
Catalase
- CCER:
-
Cerebral childhood ALD
- CEL:
-
Carboxyethyl-lysine
- CML:
-
Carboxylmethyl-lysine
- CNS:
-
Central nervous system
- DNA:
-
Deoxyribonucleic acid
- GPx:
-
Glutathione peroxidase
- GSA:
-
Glutamic semialdehyde
- GSH:
-
Glutathione
- GSHRd:
-
Glutathione reductase
- GSSG:
-
Oxidized glutathione
- H2DCFDA:
-
6-Carboxy 2′,7′-dichlorodihydrofluorescein
- H2O2 :
-
Hydrogen peroxide
- HNE:
-
4-Hydroxynonenal
- HO-1:
-
Hemoxygenase-1
- HPLC:
-
High-performance liquid chromatography
- HSCT:
-
Hematopoietic stem cell transplantation
- HTZ:
-
Heterozygote or female carriers
- IEM:
-
Inborn errors of metabolism
- IFN-γ:
-
Interferon gamma
- IL-12:
-
Interleukin 12
- IL-1β:
-
Interleukin 1 beta
- iNOS:
-
Inducible nitric oxide synthase
- LA:
-
Lipoic acid
- LDL:
-
Low-density lipoprotein
- LO:
-
Lorenzo’s oil
- MDA:
-
Malondialdehyde
- MDAL:
-
Malondialdehyde–lysine
- MnSOD:
-
Manganese–superoxide dismutase
- MRI:
-
Magnetic resonance image
- mtDNA:
-
Mitochondrial DNA
- NAC:
-
N-acetyl-cystein
- NaPA:
-
Sodium phenylacetate
- NO:
-
Oxide nitric
- O2 :
-
Oxygen
- O •−2 :
-
Superoxide radical
- OH• :
-
Hydroxyl radical
- ONOO− :
-
Peroxynitrite
- OXPHOS:
-
Oxidative phosphorylation
- PlsEtn:
-
Plasmenylethanolamine
- PUFA:
-
Polyunsaturated fatty acid
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- RS:
-
Reactive species
- SOD:
-
Superoxide dismutase
- TAR:
-
Total antioxidant reactivity
- TAS:
-
Total antioxidant status
- TBA-RS:
-
Thiobarbituric acid reactive species
- TNF-α:
-
Tumor necrosis factor alpha
- TRAP:
-
Total radical-trapping antioxidant potential
- VLCFA:
-
Very long chain fatty acidy
- VPA:
-
Valproic acid
- X-ALD:
-
X-linked adrenoleukodystrophy
References
Aksenov M, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Autrup H, Daneshvar B, Dragsted LO et al (1999) Biomarkers for exposure to ambient air pollution–comparison of carcinogen-DNA adduct levels with other exposure markers and markers for oxidative stress. Environ Health Perspect 107(3):233–238
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438. doi:10.1155/2014/360438
Barschak AG, Sitta A, Deon M et al (2006) Evidence that oxidative stress is increased in plasma from patients with maple syrup urine disease. Metab Brain Dis 21:279–286
Berger J, Gärtner J (2006) X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetics aspects. Biochim Biophys Acta 1763:1721–1732
Berger J, Pujol A, Aubourg P, Forss-Petter S (2010) Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol 20:845–856
Berger J, Forss-Petter S, Eichler FS (2014) Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 98:135–142
Bezman L, Moser AB, Raymond GV et al (2001) Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol 49:512–517
Boyd-Kimball D, Castegna A, Sultana R et al (2005) Proteomic identification of proteins oxidized by Abeta (1–42) in synaptosomes: implications for Alzheimer’s disease. Brain Res 1044:206–215
Braverman NE, Moser AB (2012) Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822(9):1442–1452
Brose RD, Avramopoulos D, Smith KD (2012) SOD2 as a potential modifier of X-linked adrenoleukodystrophy clinical phenotypes. J Neurol 259:1440–1447
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
Davies KJ (2000) Oxidative stress, antioxidant defenses, and damage removal, repair and replacement systems. IUBMB Life 50(4–5):279–289
Deon M, Wajner M, Sirtori LR et al (2006) The effect of Lorenzo’s oil on oxidative stress in X-linked adrenoleukodistrophy. J Neurol Sci 247:157–164
Deon M, Sitta A, Barschak AG et al (2007) Introduction of lipid peroxidation and decrease of antioxidant defenses in symptomatic and asymptomatic patients with X-linked adrenoleukodystrophy. Int J Dev Neurosci 25:441–444
Deon M, Sitta A, Barschak AG et al (2008) Oxidative stress is induced in female carriers of X-linked adrenoleukodystrophy. J Neurol Sci 266:79–83
Di Biase A, Salvati S, Varí R et al (2000) Susceptibility to oxidation of plasma low-density lipoprotein in X-linked adrenoleukodystrophy: effects of simvastatin treatment. Mol Genet Metab 71(4):651–655
Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32(11):1102–1115
Engelen M, Kemp S, de Visser M et al (2012) X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 7:51
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879
Fourcade S, López-Erauskin J, Galino J et al (2008) Early oxidative damage underlying neurodegenaration in X-adrenoleukodystrophy. Hum Mol Genet 17:1762–1773
Fourcade S, Ruiz M, Guilera C et al (2010) Valproic acid induces antioxidant effects in X-linked adrenoleukodystrophy. Hum Mol Genet 19:2005–2014
Fourcade S, López-Erauskin J, Ruiz M et al (2014) Mitochondrial dysfunction and oxidative damage cooperatively fuel axonal degeneration in X-linked adrenoleukodystrophy. Biochimie 98:143–149
Fridovich I (1999) Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen. Ann N Y Acad Sci 893:13–18
Galea E, Launay N, Portero-Otin M et al (2012) Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta 1822:1475–1488
Gilg GA, Singh AK, Singh I (2000) Inducible nitric oxide synthase in the central nervous system of patients with X-adrenoleukodystrophy. J Neuropathol Exp Neurol 59(12):1063–1069
Gorgas K, Teigler A, Komljenovic D, Just WW (2006) The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta 1763(12):1511–1526
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause or consequence? Lancet 344:721–724
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford University Press, Oxford
Kampa M, Nistikaki A, Tsaousis V, Maliaraki N, Notas G, Castanas E (2002) A new automated method for the determination of the total antioxidant capacity (TAC) of human plasma, based on the crocin bleaching assay. BMC Clin Pathol 2(1):1–16
Kemp S, Wanders RJA (2010) Biochemical aspects of X-linked adrenoleukodystrophy. Brain Pathol 20(4):831–837
Kemp S, Berger J, Aubourg P (2012) X-linked adrenoleukodystrophy: clinical, metabolic, genetic andpathophysiological aspects. Biochim Biophys Acta 1822:1465–1474
Khan M, Pahan K, Singh AK, Singh I (1998) Cytokine-induced accumulation of very long-chain fatty acids in rat C6 glial cells: implication for X-adrenoleukodystrophy. J Neurochem 71(1):78–87
Khan M, Singh J, Singh I (2008) Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J Neurochem 106(4):1766–1779
Kühn H, Borchert A (2002) Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med 33(2):154–172
Latorre E, Collado MP, Fernández I, Aragonés MD, Catalán RE (2003) Signaling events mediating activation of brain ethanolamine plasmalogen hydrolysis by ceramide. Eur J Biochem 270(1):36–46
Lee H-C, Wei Y-H (2005) Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 37:822–834
Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36(9):1495–1502
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Lissi E, Salim-Hanna M, Pascual C, Del Castillo MD (1995) Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Rad Biol Med 18:153–158
López-Erauskin J, Fourcade S, Galino J et al (2011) Antioxidants halt axonal neurodegeneration in a mouse model of X-adrenoleukopystrophy. Ann Neurol 70:84–92
López-Erauskin J, Galino J, Bianchi P, Fourcade S et al (2012) Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain 135(Pt12):3584–3598
López-Erauskin J, Galino J, Ruiz M et al (2013) Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum Mol Genet 22(16):3296–3305
Maier EM, Kammerer S, Muntau AC, Wichers M, Braun A, Roscher AA (2002) Symptoms in carriers of Adrenoleukodystrophy relate to skewed X inactivation. Ann Neurol 52(5):683–688
Miller DM, Buettner GR, Aust SD (1990) Transition metals as catalysts of “autoxidation” reactions. Free Radic Biol Med 8(1):95–108
Moser HW (2006) Therapy of X-linked adrenoleukodystrophy. NeuroRx 3:246–253
Moser HW, Moser AB (1991) Measurement of saturated very long chain fatty acid in plasma. In: Hommes FA (ed) Techniques in diagnostics human biochemical genetics. Wiley-Liss, New York, pp 177–191
Moser AB, Kreiter N, Bezman L et al (1999) Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol 45(1):100–110
Moser H, Smith KD, Watkins PA, Powers J, Moser AB (2001) X-linked adrenoleukodystrophy. In: Scriver CR, Sly WS (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New-York, pp 3257–3301
Moser HW, Raymond GV, Dubey P (2005a) Adrenoleukodystrophy- new approaches to a neurodegenerative disease. JAMA 294:3131–3134
Moser HW, Raymond GV, Lu SE et al (2005b) Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo’s oil. Arch Neurol 62(7):1073–1080
Negretto GW, Deon M, Burin M et al (2014) In vitro effect of genistein on DNA damage in leukocytes from mucopolysaccharidosis IVA patients. Mol Genet Metab 111(2):205–208
Niki E, Yoshida Y, Saito Y, Noguchi N (2005) Lipid peroxidation: mechanisms, inhibition, and biological effects. BBRC 338(1):668–676
Norton WT (1984) Some thoughts on the neurobiology of the leukodystrophies. Neuropediatrics 15:28–31
Ohyashiki T, Sakata N, Matsui K (1994) Changes in SH reactivity of the protein in porcine intestinal brush-border membranes associated with lipid peroxidation. J Biochem 115:224–229
Pai GS, Khan M, Barbosa E et al (2000) Lovastatin therapy for X-linked adrenoleukodystrophy: clinical and biochemical observations on 12 patients. Mol Genet Metab 69:312–322
Peters C, Charnas LR, Tan Y et al (2004) Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 104(3):881–888
Petrillo S, Piemonte F, Pastore A et al (2013) Glutathione imbalance in patients with X-linked adrenoleukodystrophy. Mol Genet Metab 109:366–370
Powers JM, Pei Z, Heinzer AK et al (2005) Adreno-leukodystrophy: oxidative stress of mice and men. J Neuropathol Exp Neurol 64:1067–1079
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288(2):481–487
Ribas GS, Manfredini V, De Mari J et al (2010) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of L-carnitine supplementation. Int J Dev Neurosci 28(2):127–132
Rockenbach FJ, Deon M, Marchese DP et al (2012) The effect of bone marrow transplantation on oxidative stress in X-linked adrenoleukodystrophy. Mol Genet Metab 106:231–236
Schofield D, Braganza JM (1996) Shortcomings of an automated assay for total antioxidant status in biological fluids. Clin Chem 42(10):1712–1714
Seifried HE, Anderson DE, Fisher EI, Milner JA (2007) A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem 18:567–579
Shichiri M (2014) The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr 54(3):151–160
Singh I, Pujol A (2010) Pathomecanisms underlying X-adrenoleukodystrophy: a three-hit hypothesis. Brain Pathol 20:838–844
Singh I, Pahan K, Khan M (1998) Lovastatin and sodium phenylacetate normalize the levels of very long chain fatty acids in skin fibroblasts of X-adrenoleukodystrophy. FEBS Lett 426:342–346
Sirtori LR, Dutra-Filho CS, Fitarelli D et al (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740:68–73
Smith KD, Kemp S, Braiterman LT et al (1999) X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem Res 24(4):521–535
Stadtman ER, Levine RL (2003) Free-radical mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
Tarpey MM, Fridovich I (2001) Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res 89(3):224–236
Tolar J, Orchard PJ, Bjoraker KJ, Ziegler RS, Shapiro EG, Charnas L (2007) N-acetyl-l-cysteine improves outcome of advanced cerebral adrenoleukodystrophy. Bone Marrow Transplant 39(4):211–215
Triantafyllou P, Economou M, Vlachaki E et al (2014) Multiple endocrine disorders associated with adrenomyeloneuropathy and a novel mutation of the ABCD1 gene. Pediatr Neurol 50(6):622–624
Uto T, Contreras MA, Gilg AG, Singh I (2008) Oxidative imbalance in nonstimulated X-adrenoleukodystrophy-derived lymphoblasts. Dev Neurosci 30:410–418
Valko M, Leibfritz D, Moncola J, Cronin M, Mazura M, Telser I (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Vargas CR, Wajner M, Sirtori LR et al (2004) Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy. Biochim Biophys Acta 1688:26–32
Wajner M, Latini A, Wyse AT, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27:427–448
Wanders RJ, Waterham HR (2006) Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta 1763(12):1707–1720
Wanders RJA, Schutgens RBH, Barth PG, Tager JM, Van Den Bosch H (1993) Postnatal diagnosis of peroxisomal disorders: a biochemical approach. Biochemie 75:269–279
Zheng M, Storz G (2000) Redox sensing by prokaryotic transcription factors. Biochem Pharmacol 59(1):1–6
Acknowledgments
The authors were supported by grants from the Brazilian Foundation Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Grant No. 007481/2011-13), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundo de Incentivo à Pesquisa e Eventos (FIPE/HCPA) (Project Nos. 01-0432, 10-0482 and 13-0247).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors (Marion Deon, Desirèe P. Marchetti, Bruna Donida, Moacir Wajner and Carmen R. Vargas) declare that there are no financial interests and/or no conflict of interest disclosure associated with this manuscript.
Ethical Standards
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Rights and permissions
About this article
Cite this article
Deon, M., Marchetti, D.P., Donida, B. et al. Oxidative Stress in Patients with X-Linked Adrenoleukodystrophy. Cell Mol Neurobiol 36, 497–512 (2016). https://doi.org/10.1007/s10571-015-0234-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0234-2