Abstract
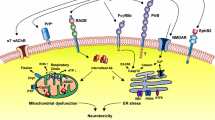
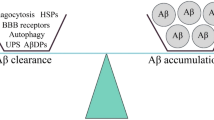
Receptor for advanced glycation end products (RAGE) is a receptor of the immunoglobulin super family that plays various important roles under physiological and pathological conditions. Compelling evidence suggests that RAGE acts as both an inflammatory intermediary and a critical inducer of oxidative stress, underlying RAGE-induced Alzheimer-like pathophysiological changes that drive the process of Alzheimer’s disease (AD). A critical role of RAGE in AD includes beta-amyloid (Aβ) production and accumulation, the formation of neurofibrillary tangles, failure of synaptic transmission, and neuronal degeneration. The steady-state level of Aβ depends on the balance between production and clearance. RAGE plays an important role in the Aβ clearance. RAGE acts as an important transporter via regulating influx of circulating Aβ into brain, whereas the efflux of brain-derived Aβ into the circulation via BBB is implemented by LRP1. RAGE could be an important contributor to Aβ generation via enhancing the activity of β- and/or γ-secretases and activating inflammatory response and oxidative stress. However, sRAGE–Aβ interactions could inhibit Aβ neurotoxicity and promote Aβ clearance from brain. Meanwhile, RAGE could be a promoting factor for the synaptic dysfunction and neuronal circuit dysfunction which are both the material structure of cognition, and the physiological and pathological basis of cognition. In addition, RAGE could be a trigger for the pathogenesis of Aβ and tau hyper-phosphorylation which both participate in the process of cognitive impairment. Preclinical and clinical studies have supported that RAGE inhibitors could be useful in the treatment of AD. Thus, an effective measure to inhibit RAGE may be a novel drug target in AD.


Similar content being viewed by others
References
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E et al (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18:484–496
Alves J, Magalhaes R, Arantes M, Cruz S, Goncalves OF, Sampaio A (2015) Cognitive rehabilitation in a visual variant of Alzheimer’s disease. Appl Neuropsychol Adult 22:73–78
Baiguera S, Fioravanzo L, Grandi C, Di LR, Parnigotto PP, Folin M (2009) Involvement of the receptor for advanced glycation-end products (RAGE) in beta-amyloid-induced toxic effects in rat cerebromicrovascular endothelial cells cultured in vitro. Int J Mol Med 24:9–15
Barroso E, Del Valle J, Porquet D, Vieira Santos AM, Salvado L, Rodriguez-Rodriguez R, Gutierrez P, Anglada-Huguet M, Alberch J, Camins A et al (2013) Tau hyperphosphorylation and increased BACE1 and RAGE levels in the cortex of PPARbeta/delta-null mice. Biochim Biophys Acta 1832:1241–1248
Bellucci A, Rosi MC, Grossi C, Fiorentini A, Luccarini I, Casamenti F (2007) Abnormal processing of tau in the brain of aged TgCRND8 mice. Neurobiol Dis 27:328–338
Bidzan M, Bidzan L (2014) Neurobehavioral manifestation in early period of Alzheimer disease and vascular dementia. Psychiatr Pol 48:319–330
Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP (2005) Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 83:876–886
Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW (2005) Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta 1739:216–223
Bomba M, Ciavardelli D, Silvestri E, Canzoniero LM, Lattanzio R, Chiappini P, Piantelli M, Di IC, Consoli A, Sensi SL (2013) Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis 4:e612
Bonetti TC, Borges E Jr, Braga DP, Iaconelli A Jr, Kleine JP, Silva ID (2013) Intrafollicular soluble receptor for advanced glycation end products (sRAGE) and embryo quality in assisted reproduction. Reprod Biomed Online 26:62–67
Caldeira GL, Ferreira IL, Rego AC (2013) Impaired transcription in Alzheimer’s disease: key role in mitochondrial dysfunction and oxidative stress. J Alzheimers Dis 34:115–131
Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G (2012) Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60:188–197
Chaney MO, Stine WB, Kokjohn TA, Kuo YM, Esh C, Rahman A, Luehrs DC, Schmidt AM, Stern D, Yan SD et al (2005) RAGE and amyloid beta interactions: atomic force microscopy and molecular modeling. Biochim Biophys Acta 1741:199–205
Chappey O, Dosquet C, Wautier MP, Wautier JL (1997) Advanced glycation end products, oxidant stress and vascular lesions. Eur J Clin Invest 27:97–108
Chavakis T, Bierhaus A, Nawroth PP (2004) RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect 6:1219–1225
Chekir C, Nakatsuka M, Noguchi S, Konishi H, Kamada Y, Sasaki A, Hao L, Hiramatsu Y (2006) Accumulation of advanced glycation end products in women with preeclampsia: possible involvement of placental oxidative and nitrative stress. Placenta 27:225–233
Chen Z, Zhong C (2013) Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol 108:21–43
Chen X, Zhang T, Du G (2008) Advanced glycation end products serve as ligands for lectin-like oxidized low-density lipoprotein receptor-1(LOX-1): biochemical and binding characterizations assay. Cell Biochem Funct 26:760–770
Chen S, An FM, Yin L, Liu AR, Yin DK, Yao WB, Gao XD (2014) Glucagon-like peptide-1 protects hippocampal neurons against advanced glycation end product-induced tau hyperphosphorylation. Neuroscience 256:137–146
Choi BR, Cho WH, Kim J, Lee HJ, Chung C, Jeon WK, Han JS (2014) Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer’s disease. Exp Mol Med 46:e75
Chuah YK, Basir R, Talib H, Tie TH, Nordin N (2013) Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam 2013:403460
Cohen MM Jr (2013) Perspectives on RAGE signaling and its role in cardiovascular disease. Am J Med Genet A 161A:2750–2755
Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N et al (2011) Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 27:3–20
Dai Y, Kamal MA (2014) Fighting Alzheimer’s disease and type 2 diabetes: pathological links and treatment strategies. CNS Neurol Disord 13:271–282
Dar TA, Sheikh IA, Ganie SA, Ali R, Singh LR, Gan SH, Kamal MA, Zargar MA (2014) Molecular linkages between diabetes and Alzheimer’s disease: current scenario and future prospects. CNS Neurol Disord 13:290–298
de la Monte SM, Tong M (2014) Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 88:548–559
de Silva R, Lashley T, Gibb G, Hanger D, Hope A, Reid A, Bandopadhyay R, Utton M, Strand C, Jowett T et al (2003) Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol 29:288–302
Di BB, Li HW, Li WP, Shen XH, Sun ZJ, Wu X (2015) Pioglitazone inhibits high glucose-induced expression of receptor for advanced glycation end products in coronary artery smooth muscle cells. Mol Med Rep 11:2601–2607
Dobarro M, Gerenu G, Ramirez MJ (2013) Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int J Neuropsychopharmacol 16:2245–2257
Durany N, Munch G, Michel T, Riederer P (1999) Investigations on oxidative stress and therapeutical implications in dementia. Eur Arch Psychiatry Clin Neurosci 249((Suppl 3)):68–73
Esposito G, Scuderi C, Lu J, Savani C, De Filippis D, Iuvone T, Steardo L Jr, Sheen V, Steardo L (2008) S100B induces tau protein hyperphosphorylation via Dickopff-1 up-regulation and disrupts the Wnt pathway in human neural stem cells. J Cell Mol Med 12:914–927
Faden AI, Loane DJ (2014) Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation. Neurotherapeutics. 12:143–150
Farmer DG, Ewart MA, Mair KM, Kennedy S (2014) Soluble receptor for advanced glycation end products (sRAGE) attenuates haemodynamic changes to chronic hypoxia in the mouse. Pulm Pharmacol Ther 29:7–14
Fernandez JA, Rojo L, Kuljis RO, Maccioni RB (2008) The damage signals hypothesis of Alzheimer’s disease pathogenesis. J Alzheimers Dis 14:329–333
Franko B, Brault J, Jouve T, Beaumel S, Benhamou PY, Zaoui P, Stasia MJ (2014) Differential impact of glucose levels and advanced glycation end-products on tubular cell viability and pro-inflammatory/profibrotic functions. Biochem Biophys Res Commun. 451:627–631
Fuster-Matanzo A, Llorens-Martin M, Hernandez F, Avila J (2013) Role of neuroinflammation in adult neurogenesis and Alzheimer disease: therapeutic approaches. Mediators Inflamm 2013:260925
Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS (2014) Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology 82:1536–1542
Gasparotto J, Senger MR, Kunzler A, Degrossoli A, de Simone SG, Bortolin RC, Somensi N, Girardi CS, de Souza Cda S, Calabrese KS et al (2015) Increased tau phosphorylation and receptor for advanced glycation endproducts (RAGE) in the brain of mice infected with Leishmania amazonensis. Brain Behav Immun 43:37–45
Heilmann RM, Otoni CC, Jergens AE, Grutzner N, Suchodolski JS, Steiner JM (2014) Systemic levels of the anti-inflammatory decoy receptor soluble RAGE (receptor for advanced glycation end products) are decreased in dogs with inflammatory bowel disease. Vet Immunol Immunopathol 161:184–192
Hoyer S (2000) Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: an update. Exp Gerontol 35:1363–1372
Iacono D, Resnick SM, O’Brien R, Zonderman AB, An Y, Pletnikova O, Rudow G, Crain B, Troncoso JC (2014) Mild cognitive impairment and asymptomatic Alzheimer disease subjects: equivalent beta-amyloid and tau loads with divergent cognitive outcomes. J Neuropathol Exp Neurol 73:295–304
Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM (2013) Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci 33:1024–1037
Isono T, Yamashita N, Obara M, Araki T, Nakamura F, Kamiya Y, Alkam T, Nitta A, Nabeshima T, Mikoshiba K et al (2013) Amyloid-beta(2)(5)(-)(3)(5) induces impairment of cognitive function and long-term potentiation through phosphorylation of collapsin response mediator protein 2. Neurosci Res 77:180–185
Kalousova M, Zima T (2014) AGEs and RAGE—advanced glycation end-products and their receptor in questions and answers. Vnitr Lek 60:720–724
Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 10:187–198
Kenessey A, Nacharaju P, Ko LW, Yen SH (1997) Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J Neurochem 69:2026–2038
Kook SY, Seok HH, Moon M, Mook-Jung I (2013) Disruption of blood-brain barrier in Alzheimer disease pathogenesis. Tissue Barriers 1:e23993
Kosenko EA, Solomadin IN, Tikhonova LA, Reddy VP, Aliev G, Kaminsky YG (2014) Pathogenesis of Alzheimer disease: role of oxidative stress, amyloid-beta peptides, systemic ammonia and erythrocyte energy metabolism. CNS Neurol Disord 13:112–119
Krautwald M, Munch G (2010) Advanced glycation end products as biomarkers and gerontotoxins—A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease. Exp Gerontol 45:744–751
Kuhla A, Ludwig SC, Kuhla B, Munch G, Vollmar B (2014) Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer’s disease brain. Neurobiol Aging 36:753–761
Kuwahara H, Nishida Y, Yokota T (2013) Blood-brain barrier and Alzheimer’s disease. Brain and nerve = Shinkei kenkyu no shinpo 65:145–151
Kvartsberg H, Duits FH, Ingelsson M, Andreasen N, Ohrfelt A, Andersson K, Brinkmalm G, Lannfelt L, Minthon L, Hansson O, et al (2014) Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement
Lee G, Cowan N, Kirschner M (1988) The primary structure and heterogeneity of tau protein from mouse brain. Science 239:285–288
Lee YS, Kim H, Kim YH, Roh EJ, Han H, Shin KJ (2012) Synthesis and structure-activity relationships of tri-substituted thiazoles as RAGE antagonists for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 22:7555–7561
Leuner K, Muller WE, Reichert AS (2012) From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol 46:186–193
Li J, Liu D, Sun L, Lu Y, Zhang Z (2012a) Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci 317:1–5
Li K, Dai D, Zhao B, Yao L, Yao S, Wang B, Yang Z (2010) Association between the RAGE G82S polymorphism and Alzheimer's disease. J Neural Transm 117:97–104
Li XH, Lv BL, Xie JZ, Liu J, Zhou XW, Wang JZ (2012b) AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol Aging 33:1400–1410
Li XH, Xie JZ, Jiang X, Lv BL, Cheng XS, Du LL, Zhang JY, Wang JZ, Zhou XW (2012c) Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. Neuromolecular Med. 14:338–348
Li XH, Du LL, Cheng XS, Jiang X, Zhang Y, Lv BL, Liu R, Wang JZ, Zhou XW (2013) Glycation exacerbates the neuronal toxicity of beta-amyloid. Cell Death Dis 4:e673
Liang F, Jia J, Wang S, Qin W, Liu G (2013) Decreased plasma levels of soluble low density lipoprotein receptor-related protein-1 (sLRP) and the soluble form of the receptor for advanced glycation end products (sRAGE) in the clinical diagnosis of Alzheimer’s disease. J Clin Neurosci 20:357–361
Liu R, Wu CX, Zhou D, Yang F, Tian S, Zhang L, Zhang TT, Du GH (2012) Pinocembrin protects against beta-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med 10:105
Liu R, Li JZ, Song JK, Zhou D, Huang C, Bai XY, Xie T, Zhang X, Li YJ, Wu CX et al (2014) Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiol Aging 35:1275–1285
Lue LF, Walker DG, Jacobson S, Sabbagh M (2009) Receptor for advanced glycation end products: its role in Alzheimer’s disease and other neurological diseases. Future Neurol 4:167–177
Lukiw WJ (2012) NF-kappaB-regulated, proinflammatory miRNAs in Alzheimer’s disease. Alzheimers Res Ther 4:47
Luth HJ, Ogunlade V, Kuhla B, Kientsch-Engel R, Stahl P, Webster J, Arendt T, Munch G (2005) Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb Cortex 15:211–220
Lv C, Wang L, Liu X, Yan S, Yan SS, Wang Y, Zhang W (2015) Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 89:175–184
Mahboobi H, Golmirzaei J, Gan SH, Jalalian M, Kamal MA (2014) Humanin: a possible linkage between Alzheimer’s disease and type 2 diabetes. CNS Neurol Disord 13:543–552
Marksteiner J, Imarhiagbe D, Defrancesco M, Deisenhammer EA, Kemmler G, Humpel C (2014) Analysis of 27 vascular-related proteins reveals that NT-proBNP is a potential biomarker for Alzheimer’s disease and mild cognitive impairment: a pilot-study. Exp Gerontol 50:114–121
Matrone C, Djelloul M, Taglialatela G, Perrone L (2015) Inflammatory risk factors and pathologies promoting Alzheimer’s disease progression: is RAGE the key. Histol Histopathol 30:125–139
Moriya S, Yamazaki M, Murakami H, Maruyama K, Uchiyama S (2014) Two soluble isoforms of receptors for advanced glycation end products (RAGE) in carotid atherosclerosis: the difference of soluble and endogenous secretory RAGE. J Stroke Cerebrovasc Dis 23:2540–2546
Motoyoshi S, Yamamoto Y, Munesue S, Igawa H, Harashima A, Saito H, Han D, Watanabe T, Sato H, Yamamoto H (2014) cAMP ameliorates inflammation by modulation of macrophage receptor for advanced glycation end-products. Biochem J 463:75–82
Munch G, Thome J, Foley P, Schinzel R, Riederer P (1997) Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res Brain Res Rev 23:134–143
Opatrna S, Popperlova A, Kalousova M, Zima T (2014) Low glucose degradation product peritoneal dialysis regimen is associated with lower plasma EN-RAGE and HMGB-1 proinflammatory ligands of receptor for advanced glycation end products. Ther Apher Dial 18:309–316
Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, Mufson EJ (2013) Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J Comp Neurol 521:4318–4338
Perrone L, Grant WB (2015) Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J Alzheimers Dis 45:965–979
Pertynska-Marczewska M, Merhi Z (2014) Relationship of advanced glycation end products with cardiovascular disease in menopausal women. Reprod Sci. 22:447–482
Piras S, Furfaro AL, Piccini A, Passalacqua M, Borghi R, Carminati E, Parodi A, Colombo L, Salmona M, Pronzato MA et al (2014) Monomeric Abeta1-42 and RAGE: key players in neuronal differentiation. Neurobiol Aging 35:1301–1308
Polydoro M, de Calignon A, Suarez-Calvet M, Sanchez L, Kay KR, Nicholls SB, Roe AD, Pitstick R, Carlson GA, Gomez-Isla T et al (2013) Reversal of neurofibrillary tangles and tau-associated phenotype in the rTgTauEC model of early Alzheimer’s disease. J Neurosci 33:13300–13311
Porsteinsson AP, Keltz MA, Smith JS (2014) Role of citalopram in the treatment of agitation in Alzheimer’s disease. Neurodegener Dis Manag 4:345–349
Pozueta J, Lefort R, Shelanski ML (2013) Synaptic changes in Alzheimer’s disease and its models. Neuroscience 251:51–65
Provias J, Jeynes B (2014) The role of the blood-brain barrier in the pathogenesis of senile plaques in Alzheimer’s disease. Int J Alzheimers Dis 2014:191863
Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J (2014) Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex—evidence from two transgenic mouse models. Alzheimers Res Ther 6:2
Qosa H, LeVine H 3rd, Keller JN, Kaddoumi A (2014) Mixed oligomers and monomeric amyloid-beta disrupts endothelial cells integrity and reduces monomeric amyloid-beta transport across hCMEC/D3 cell line as an in vitro blood-brain barrier model. Biochim Biophys Acta 1842:1806–1815
Quade-Lyssy P, Kanarek AM, Baiersdorfer M, Postina R, Kojro E (2013) Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. J Lipid Res 54:3052–3061
Qureshi HY, Li T, MacDonald R, Cho CM, Leclerc N, Paudel HK (2013) Interaction of 14-3-3zeta with microtubule-associated protein tau within Alzheimer’s disease neurofibrillary tangles. Biochemistry 52:6445–6455
Ramamurthy B, Larsson L (2013) Detection of an aging-related increase in advanced glycation end products in fast- and slow-twitch skeletal muscles in the rat. Biogerontology 14:293–301
Rapoport SI, Hatanpaa K, Brady DR, Chandrasekaran K (1996) Brain energy metabolism, cognitive function and down-regulated oxidative phosphorylation in Alzheimer disease. Neurodegeneration 5:473–476
Rofina JE, Singh K, Skoumalova-Vesela A, van Ederen AM, van Asten AJ, Wilhelm J, Gruys E (2004) Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid 11:90–100
Sabbagh MN, Agro A, Bell J, Aisen PS, Schweizer E, Galasko D (2011) PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer disease. Alzheimer Dis Assoc Disord 25:206–212
Salahuddin P, Rabbani G, Khan RH (2014) The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cell Mol Biol Lett 19:407–437
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118:460–474
Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, Koike T, Wakayama I, Yanagihara R, Garruto R et al (1998) Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol 153:1149–1155
Sato N, Takeda S, Uchio-Yamada K, Ueda H, Fujisawa T, Rakugi H, Morishita R (2011) Role of insulin signaling in the interaction between Alzheimer disease and diabetes mellitus: a missing link to therapeutic potential. Curr Aging Sci 4:118–127
Schmidt ML, Huang R, Martin JA, Henley J, Mawal-Dewan M, Hurtig HI, Lee VM, Trojanowski JQ (1996) Neurofibrillary tangles in progressive supranuclear palsy contain the same tau epitopes identified in Alzheimer’s disease PHFtau. J Neuropathol Exp Neurol 55:534–539
Semba RD, Gebauer SK, Baer DJ, Sun K, Turner R, Silber HA, Talegawkar S, Ferrucci L, Novotny JA (2014) Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr 144:1037–1042
Sharma HS, Castellani RJ, Smith MA, Sharma A (2012) The blood-brain barrier in Alzheimer’s disease: novel therapeutic targets and nanodrug delivery. Int Rev Neurobiol 102:47–90
Shemirani F, Yazdanparast R (2014) The interplay between hyperglycemia-induced oxidative stress markers and the level of soluble receptor for advanced glycation end products (sRAGE) in K562 cells. Mol Cell Endocrinol 393:179–186
Shin J, Lee SY, Kim SH, Kim YB, Cho SJ (2008) Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. Neuroimage 43:236–244
Shin S, Kim JH, Cho JH, Kim GS, Choi SA, Lee JH (2014) Mild cognitive impairment due to Alzheimer disease is less likely under the age of 65. Alzheimer Dis Assoc Disord 29:26–31
Slowik A, Merres J, Elfgen A, Jansen S, Mohr F, Wruck CJ, Pufe T, Brandenburg LO (2012) Involvement of formyl peptide receptors in receptor for advanced glycation end products (RAGE)—and amyloid beta 1-42-induced signal transduction in glial cells. Mol Neurodegener 7:55
Smith MA, Monnier VM, Sayre LM, Perry G (1995) Amyloidosis, advanced glycation end products and Alzheimer disease. NeuroReport 6:1595–1596
Son SM, Jung ES, Shin HJ, Byun J, Mook-Jung I (2012) Abeta-induced formation of autophagosomes is mediated by RAGE-CaMKKbeta-AMPK signaling. Neurobiol Aging 33:1006 e11–23
Steele JW, Brautigam H, Short JA, Sowa A, Shi M, Yadav A, Weaver CM, Westaway D, Fraser PE, St GPH et al (2014) Early fear memory defects are associated with altered synaptic plasticity and molecular architecture in the TgCRND8 Alzheimer’s disease mouse model. J Comp Neurol 522:2319–2335
Stitt AW, Jenkins AJ, Cooper ME (2002) Advanced glycation end products and diabetic complications. Expert Opin Investig Drugs 11:1205–1223
Sugihara T, Munesue S, Yamamoto Y, Sakurai S, Akhter N, Kitamura Y, Shiba K, Watanabe T, Yonekura H, Hayashi Y et al (2012) Endogenous secretory receptor for advanced glycation end-products inhibits amyloid-beta1-42 uptake into mouse brain. J Alzheimers Dis 28:709–720
Tabaton M, Cammarata S, Manetto V, Perry G, Mancardi G (1989) Tau-reactive neurofibrillary tangles in cerebellar cortex from patients with Alzheimer’s disease. Neurosci Lett 103:259–262
Takeuchi M, Yamagishi S (2008) Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr Pharm Des 14:973–978
Takeuchi M, Kikuchi S, Sasaki N, Suzuki T, Watai T, Iwaki M, Bucala R, Yamagishi S (2004) Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr Alzheimer Res 1:39–46
Takeuchi M, Sato T, Takino J, Kobayashi Y, Furuno S, Kikuchi S, Yamagishi S (2007) Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer’s disease. Med Hypotheses 69:1358–1366
Tancharoen S, Tengrungsun T, Suddhasthira T, Kikuchi K, Vechvongvan N, Tokuda M, Maruyama I (2014) Overexpression of receptor for advanced glycation end products and high-mobility group box 1 in human dental pulp inflammation. Mediators Inflamm 2014:754069
Tangen GG, Engedal K, Bergland A, Moger TA, Mengshoel AM (2014) Relationships between balance and cognition in patients with subjective cognitive impairment, mild cognitive impairment, and Alzheimer disease. Phys Ther 94:1123–1134
Thomas MC, Forbes JM, Cooper ME (2005) Advanced glycation end products and diabetic nephropathy. Am J Ther 12:562–572
Thome J, Kornhuber J, Munch G, Schinzel R, Taneli Y, Zielke B, Rosler M, Riederer P (1996) New hypothesis on etiopathogenesis of Alzheimer syndrome. Advanced glycation end products (AGEs). Nervenarzt 67:924–929
Tomoo K, Yao TM, Minoura K, Hiraoka S, Sumida M, Taniguchi T, Ishida T (2005) Possible role of each repeat structure of the microtubule-binding domain of the tau protein in in vitro aggregation. J Biochem 138:413–423
Valente T, Gella A, Fernandez-Busquets X, Unzeta M, Durany N (2010) Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol Dis 37:67–76
Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno AI (2012) Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int J Immunopathol Pharmacol 25:345–353
Vicente MH, Outeiro TF (2010) The sour side of neurodegenerative disorders: the effects of protein glycation. J. Pathol. 221:13–25
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA 91:4766–4770
Wan W, Chen H, Li Y (2014) The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer’s disease. Int J Neurosci 124:75–81
Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X, Li Y, Xia S (2015) Abeta oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem. doi:10.1111/jnc.13122
Wang CY, Xie JW, Xu Y, Wang T, Cai JH, Wang X, Zhao BL, An L, Wang ZY (2013) Trientine reduces BACE1 activity and mitigates amyloidosis via the AGE/RAGE/NF-kappaB pathway in a transgenic mouse model of Alzheimer’s disease. Antioxid Redox Signal 19:2024–2039
Wang X, Yu S, Hu JP, Wang CY, Wang Y, Liu HX, Liu YL (2014) Streptozotocin-induced diabetes increases amyloid plaque deposition in AD transgenic mice through modulating AGEs/RAGE/NF-kappaB pathway. Int J Neurosci 124:601–608
Wautier MP, Tessier FJ, Wautier JL (2014) Advanced glycation end products: a risk factor for human health. Ann Pharm Fr 72:400–408
Webster B, Hansen L, Adame A, Crews L, Torrance M, Thal L, Masliah E (2006) Astroglial activation of extracellular-regulated kinase in early stages of Alzheimer disease. J Neuropathol Exp Neurol 65:142–151
Xiao Z, Cilz NI, Kurada L, Hu B, Yang C, Wada E, Combs CK, Porter JE, Lesage F, Lei S (2014) Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: beneficial actions in an Alzheimer’s disease model. J Neurosci 34:7027–7042
Xie C, Miyasaka T, Yoshimura S, Hatsuta H, Yoshina S, Kage-Nakadai E, Mitani S, Murayama S, Ihara Y (2014) The homologous carboxyl-terminal domains of microtubule-associated protein 2 and TAU induce neuronal dysfunction and have differential fates in the evolution of neurofibrillary tangles. PLoS ONE 9:e89796
Xu L, Zang P, Feng B, Qian Q (2014) Atorvastatin inhibits the expression of RAGE induced by advanced glycation end products on aortas in healthy Sprague-Dawley rats. Diabetol Metab Syndr 6:102
Xue J, Ray R, Singer D, Bohme D, Burz DS, Rai V, Hoffmann R, Shekhtman A (2014) The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry 53:3327–3335
Yamagishi SI, Fukami K, Matsui T (2015) Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc Diabetol 14:2
Yamagishi S, Matsui T (2010) Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front Biosci (Elite Ed) 2:1184–1195
Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA et al (1994) Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci USA 91:7787–7791
Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P et al (1995) Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med 1:693–699
Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J et al (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382:685–691
Yan SS, Chen D, Yan S, Guo L, Du H, Chen JX (2012) RAGE is a key cellular target for Abeta-induced perturbation in Alzheimer’s disease. Front Biosci (Schol Ed) 4:240–250
Yang Y, Song W (2013) Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 250:140–150
Yoon SS, Jo SA (2012) Mechanisms of amyloid-beta peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol Ther (Seoul) 20:245–255
Yu SL, Wong CK, Szeto CC, Li EK, Cai Z, Tam LS (2014) Members of the receptor for advanced glycation end products axis as potential therapeutic targets in patients with lupus nephritis. Lupus 24:675–686
Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, Ding SM, Wu C, Jia XB (2014) The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol 151:591–600
Zhou WW, Lu S, Su YJ, Xue D, Yu XL, Wang SW, Zhang H, Xu PX, Xie XX, Liu RT (2014) Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with Alzheimer disease. Free Radic Biol Med 74:50–63
Zong H, Madden A, Ward M, Mooney MH, Elliott CT, Stitt AW (2010) Homodimerization is essential for the receptor for advanced glycation end products (RAGE)-mediated signal transduction. J Biol Chem 285:23137–23146
Acknowledgments
This work was supported by the Hubei Province Health and Family Planning Scientific Research Project (WJ2015MB219), the Hubei Provincial Nature Science Foundation (2105CFB260) and the Nature Science Foundation of Shiyan Renmin Hospital to Dr. Zhiyou Cai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Cai, Z., Liu, N., Wang, C. et al. Role of RAGE in Alzheimer’s Disease. Cell Mol Neurobiol 36, 483–495 (2016). https://doi.org/10.1007/s10571-015-0233-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0233-3