Abstract
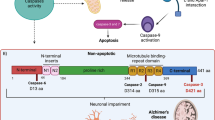
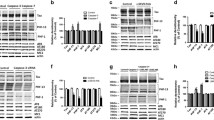
Tau truncation is widely detected in Alzheimer’s disease brain. Caspases activation is suggested to play a significant role in tau truncation at Aspartate 421 (D421) according to their ability to cleave recombinant tau in vitro. Ample evidence has shown that caspase-6 is involved in cognitive impairment and expressed in AD brain. Reactive oxygen species (ROS) can lead to caspase-6 activation and correlate with AD. Here, we transfected human embryonic kidney 293 (HEK 293) cells with Tau 441 plasmid and investigated the role of caspase-6 and caspase-3 in ROS-mediated tau truncation. Our data demonstrated that H2O2 induced oxidative stress and increased tau truncation. Caspase-6 and caspase-3 activity also increased in a dose-dependent manner in HEK 293/Tau cells during H2O2 insult. When cells were treated with an ROS inhibitor N-acetyl-l-cysteine, tau truncation was significantly suppressed. Compared with H2O2 (100 μM)/non-inhibitor group or single-inhibitor groups (z-VEID-fmk, caspase-6 inhibitor or z-DEVD-fmk, and caspase-3 inhibitor), tau truncation induced by H2O2 was effectively reduced in the combinative inhibitors group. Similar results were shown when cells were transfected with specific caspase-3 and caspase-6 siRNA. Inhibition of caspase-6 led to decline of caspase-3 activation. Taken together, our results suggest that the combination of caspase-6 and caspase-3 aggravates tau truncation at D421 induced by H2O2. Caspase-6 may play an important part in activating caspase-3. Further investigation of how the synergic role of caspase-6 and caspase-3 affects tau truncation may provide new visions for potential AD therapies.





Similar content being viewed by others
References
Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC (2007) Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol 170(4):1200–1209
Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci 22(21):9340–9351
Allsopp TE, McLuckie J, Kerr LE, Macleod M, Sharkey J, Kelly JS (2000) Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ 7(10):984–993
Basurto-Islas G, Luna-Munoz J, Guillozet-Bongaarts AL, Binder LI, Mena R, Garcia-Sierra F (2008) Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J Neuropathol Exp Neurol 67(5):470–483
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33(1):95–130
Cao XH, Wang AH, Wang CL, Mao DZ, Lu MF, Cui YQ, Jiao RZ (2010) Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem Biol Interact 183(3):357–362
Chen S, Ge X, Chen Y, Lv N, Liu Z, Yuan W (2013) Advances with RNA interference in Alzheimer’s disease research. Drug Des Devel Ther 7:117–125
Chuang CY, Chen TL, Cherng YG, Tai YT, Chen TG, Chen RM (2011) Lipopolysaccharide induces apoptotic insults to human alveolar epithelial A549 cells through reactive oxygen species-mediated activation of an intrinsic mitochondrion-dependent pathway. Arch Toxicol 85(3):209–218
Cotman CW, Poon WW, Rissman RA, Blurton-Jones M (2005) The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol 64(2):104–112
Feng Y, Xia Y, Yu G, Shu X, Ge H, Zeng K, Wang J, Wang X (2013) Cleavage of GSK-3beta by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3beta activity induced by H2O2. J Neurochem 126(2):234–242
Ferrer I, Lopez E, Blanco R, Rivera R, Krupinski J, Marti E (2000) Differential c-Fos and caspase expression following kainic acid excitotoxicity. Acta Neuropathol 99(3):245–256
Filipcik P, Cente M, Krajciova G, Vanicky I, Novak M (2009) Cortical and hippocampal neurons from truncated tau transgenic rat express multiple markers of neurodegeneration. Cell Mol Neurobiol 29(6–7):895–900
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100(17):10032–10037
Garcia-Sierra F, Mondragon-Rodriguez S, Basurto-Islas G (2008) Truncation of tau protein and its pathological significance in Alzheimer’s disease. J Alzheimers Dis 14(4):401–409
Graham RK, Ehrnhoefer DE, Hayden MR (2011) Caspase-6 and neurodegeneration. Trends Neurosci 34(12):646–656
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83(13):4913–4917
Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI (2005) Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging 26(7):1015–1022
Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol 165(2):523–531
Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B (1986) Defective brain microtubule assembly in Alzheimer’s disease. Lancet 2(8504):421–426
Jhumka Z, Pervaiz S, Clement MV (2009) Resveratrol regulates the expression of NHE-1 by repressing its promoter activity: critical involvement of intracellular H2O2 and caspases 3 and 6 in the absence of cell death. Int J Biochem Cell Biol 41(4):945–956
Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA 83(11):4044–4048
Kumar AP, Chang MK, Fliegel L, Pervaiz S, Clement MV (2007) Oxidative repression of NHE1 gene expression involves iron-mediated caspase activity. Cell Death Differ 14(10):1733–1746
LeBlanc AC (2013) Caspase-6 as a novel early target in the treatment of Alzheimer’s disease. Eur J Neurosci 37(12):2005–2018
Lee VM, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251(4994):675–678
Liu X, Kim CN, Pohl J, Wang X (1996) Purification and characterization of an interleukin-1beta-converting enzyme family protease that activates cysteine protease P32 (CPP32). J Biol Chem 271(23):13371–13376
Milton NG (2004) Role of hydrogen peroxide in the aetiology of Alzheimer’s disease: implications for treatment. Drugs Aging 21(2):81–100
Novak M, Kabat J, Wischik CM (1993) Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J 12(1):365–370
Pop C, Salvesen GS (2009) Human caspases: activation, specificity, and regulation. J Biol Chem 284(33):21777–21781
Quintanilla RA, Dolan PJ, Jin YN, Johnson GV (2012) Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging 33(3):619–635
Ramcharitar J, Albrecht S, Afonso VM, Kaushal V, Bennett DA, Leblanc AC (2013) Cerebrospinal fluid tau cleaved by caspase-6 reflects brain levels and cognition in aging and Alzheimer disease. J Neuropathol Exp Neurol 72(9):824–832
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114(1):121–130
Shimohama S, Tanino H, Fujimoto S (2001) Differential expression of rat brain caspase family proteins during development and aging. Biochem Biophys Res Commun 289(5):1063–1066
Troy CM, Salvesen GS (2002) Caspases on the brain. J Neurosci Res 69(2):145–150
Vina J, Lloret A, Orti R, Alonso D (2004) Molecular bases of the treatment of Alzheimer’s disease with antioxidants: prevention of oxidative stress. Mol Aspects Med 25(1–2):117–123
Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW (1999) Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J 18(8):2049–2056
Zhang Q, Zhang X, Chen J, Miao Y, Sun A (2009a) Role of caspase-3 in tau truncation at D421 is restricted in transgenic mouse models for tauopathies. J Neurochem 109(2):476–484
Zhang Q, Zhang X, Sun A (2009b) Truncated tau at D421 is associated with neurodegeneration and tangle formation in the brain of Alzheimer transgenic models. Acta Neuropathol 117(6):687–697
Acknowledgments
We are grateful to Dr. Jianzhi Wang (Huazhong University of Science and Technology, Wuhan, China) for the plasmid of longest human tau. This work was supported by grants from the National Natural Science Foundation of China (30970685, 81270432).
Conflict of interest
No conflicts of interest were declared.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, H., Zhao, W., Lok, K. et al. A Synergic Role of Caspase-6 and Caspase-3 in Tau Truncation at D421 Induced by H2O2 . Cell Mol Neurobiol 34, 369–378 (2014). https://doi.org/10.1007/s10571-013-0021-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-0021-x