Abstract
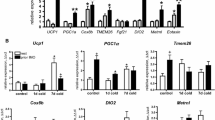
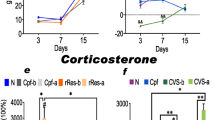
The sympathoadrenal system is the main source of catecholamines (CAs) in adipose tissues and therefore plays the key role in the regulation of adipose tissue metabolism. We recently reported existence of an alternative CA-producing system directly in adipose tissue cells, and here we investigated effect of various stressors—physical (cold) and emotional stress (immobilization) on dynamics of this system. Acute or chronic cold exposure increased intracellular norepinephrine (NE) and epinephrine (EPI) concentration in isolated rat mesenteric adipocytes. Gene expression of CA biosynthetic enzymes did not change in adipocytes but was increased in stromal vascular fraction (SVF) after 28 day cold. Exposure of rats to a single IMO stress caused increases in NE and EPI levels, and also gene expression of CA biosynthetic enzymes in adipocytes. In SVF changes were similar but more pronounced. Animals adapted to a long-term cold exposure (28 days, 4°C) did not show those responses found after a single IMO stress either in adipocytes or SVF. Our data indicate that gene machinery accommodated in adipocytes, which is able to synthesize NE and EPI de novo, is significantly activated by stress. Cold-adapted animals keep their adaptation even after an exposure to a novel stressor. These findings suggest the functionality of CAs produced endogenously in adipocytes. Taken together, the newly discovered CA synthesizing system in adipocytes is activated in stress situations and might significantly contribute to regulation of lipolysis and other metabolic or thermogenetic processes.









Similar content being viewed by others
References
Astori G, Vignati F, Bardelli S, Tubio M, Gola M, Albertini V, Bambi F, Scali G, Castelli D, Rasini V, Soldati GT, Moccetti T (2007) “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med 5:55
Bartness TJ, Bamshad M (1998) Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol 275:R1399–R1411
Bartness TJ, Song CK (2007) Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48:1655–1672
Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK (2010) Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318:34–43
Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell′Omo G, Parmigiani S, Palanza P (2009) Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS ONE 4:e4331
Bergquist J, Ohlsson B, Tarkowski A (2000) Nuclear factor-kappa B is involved in the catecholaminergic suppression of immunocompetent cells. Ann N Y Acad Sci 917:281–289
Berlan M, Lafontan M (1982) The alpha 2-adrenergic receptor on human fat cells: comparative study of alpha 2-adrenergic radioligand binding and biological response. J Physiol (Paris) 78:279–287
Bottner A, Haidan A, Eisenhofer G, Kristensen K, Castle AL, Scherbaum WA, Schneider H, Chrousos GP, Bornstein SR (2001) Increased body fat mass and suppression of circulating leptin levels in response to hypersecretion of epinephrine in phenylethanolamine-N-methyltransferase (PNMT)-overexpressing mice. Endocrinology 141:4239–4246
Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ (2004) Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286:R1167–R1175
Brito NA, Brito MN, Bartness TJ (2008) Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Interg Comp Physiol 294:R1445–R1452
Buu NT (1993) Uptake of 1-methyl-4-phenylpyridinium and dopamine in the mouse brain cell nuclei. J Neurochem 61:1557–1560
Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, Andre M, Casteilla L, Penicaud L (2005) Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett 579:3487–3492
Dronjak S, Ondriska M, Svetlovska D, Jezova D, Kvetnansky R (2002) Effects of novel stressors on plasma catecholamine levels in rats exposed to long-term cold. In: McCarty R, Aguilera G, Sabban EL, Kvetnansky R (eds) Stress neural, endocrine and molecular studies. Taylor and Francis, London/New York, pp 83–89
Engler KL, Rudd ML, Ryan JJ, Stewart JK, Fischer-Stenger K (2005) Autocrine actions of macrophage-derived catecholamines on interleukin-1 beta. J Neuroimmunol 160:87–91
Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K (2009) Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg 124(4):1087–1097
Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA (2008) Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box? Mol Med 14:195–204
Giordano A, Frontini A, Murano I, Tonello C, Marino MA, Carruba MO, Nisoli E, Cinti S (2005) Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem 53:679–687
Goldstein DS, Kopin IJ (2008) Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul 42:111–119
Guo NN, Li BM (2007) Cellular and subcellular distributions of β1- and β2-adrenoceptors in the CA1 and CA3 regions of the rat hippocampus. Neuroscience 146:298–305
Hökfelt B (1951) Noradrenaline and adrenaline in mammalian tissues. Acta Physiol Scand 25(92):134 Suppl 92
Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z (2007) Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13(7):803–811
Kvetnansky R, Mikulaj L (1970) Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 87:738–743
Kvetnansky R, Weise VK, Gewirtz GP, Kopin IJ (1971a) Synthesis of adrenal catecholamines in rats during and after immobilization stress. Endocrinology 89:46–49
Kvetnansky R, Gewirtz GP, Weise VK, Kopin IJ (1971b) Catecholamine-synthesizing enzymes in the rat adrenal gland during exposure to cold. Am J Physiol 220:928–931
Kvetnansky R, Palkovits M, Mitro A, Torda T, Mikulaj L (1977) Catecholamines in individual hypothalamic nuclei of acutely and repeatedly stressed rats. Neuroendocrinology 23:257–267
Kvetnansky R, Sun CL, Lake CR, Thoa N, Torda T, Kopin IJ (1978) Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology 103:1868–1874
Kvetnansky R, Weise VK, Thoa NB, Kopin IJ (1979) Effects of chronic guanethidine treatment and adrenal medullectomy on plasma levels of catecholamines and corticosterone in forcibly immobilized rats. J Pharmacol Exp Ther 209:287–291
Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ (1995) Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann N Y Acad Sci 771:131–158
Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Goldstein DS (1998) Stressor specificity of peripheral catecholaminergic activation. Adv Pharmacol 42:556–560
Kvetnansky R, Jelokova J, Rusnak M, Dronjak S, Serova L, Nankova B, Sabban EL (2002) Novel stressors exaggerate tyrosine hydroxylase gene expression in the adrenal medulla of rats exposed to long-term cold stress. In: McCarty R, Aguilera G, Sabban EL, Kvetnansky R (eds) Stress neural, endocrine and molecular studies. Taylor and Francis, London/New York, pp 121–128
Kvetnansky R, Micutkova L, Rychkova N, Kubovcakova L, Mravec B, Filipenko M, Sabban EL, Krizanova O (2004) Quantitative evaluation of catecholamine enzymes gene expression in adrenal medulla and sympathetic ganglia of stressed rats. Ann N Y Acad Sci 1018:356–369
Kvetnansky R, Sabban EL, Palkovits M (2009) Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89:535–606
Lafontan M, Berlan M (1995) Fat cell alpha2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr Rev 16:716–738
Lafontan M, Langin D (2009) Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48:275–297
Laukova M, Vargovic P, Krizanova O, Kvetnansky R (2010) Repeated stress down-regulates beta 2- and alpha 2C-adrenergic receptors and up-regulates gene expression of IL-6 in the rat spleen. Cell Mol Neurobiol 30:1077–1087
Laukova M, Vargovic P, Csaderova L, Chovanova L, Vlcek M, Imrich R, Krizanova O, Kvetnansky R (2012) Acute stress differently modulates beta 1, beta 2 and beta 3 adrenoceptors in T cells, but not in B cells, from the rat spleen. Neuroimmunomodulation 19:69–78
Lenartowski R, Goc A (2011) Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int J Dev Neurosci 29(8):873–883
Lencesova L, Sirova M, Csaderova L, Laukova M, Sulova Z, Kvetnansky R, Krizanova O (2010) Changes and role of adrenoceptors in PC12 cells after phenylephrine administration and apoptosis induction. Neurochem Int 57:884–892
Lin G, Garcia M, Ning H, Banie L, Guo Y-L, Lue TF, Lin C-S (2008) Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17:1053–1064
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Morton RE, Evans TA (1992) Modification of the bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal Biochem 204:332–334
Nakashima A, Hayashi N, Kanek YS, Mori K, Sabban EL, Nagatsu T, Ota A (2007) RNAi of 14-3-3eta protein increases intracellular stability of tyrosine hydroxylase. Biochem Biophys Res Commun 363(3):817–821
Nankova B, Kvetnansky R, McMahon A, Viskupic E, Hiremagalu B, Frankle G, Fukuhara K, Kopin IJ, Sabban EL (1994) Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci USA 91(13):5937–5941
Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–109
Panayotacopoulou MT, Malidelis Y, van Heerikhuize J, Unmehopa U, Swaab D (2005) Individual differences in the expression of tyrosine hydroxylase mRNA in neurosecretory neurons of the human paraventricular and supraoptic nuclei: positive correlation with vasopressin mRNA. Neuroendocrilogy 81(5):329–338
Pendleton RG, Gessner G, Sawyer J (1978) Studies on the distribution of phenylethanolamine N-methyltransferase and epinephrine in the rat. Res Commun Chem Pathol Pharmacol 21:315–325
Pizzinat N, Marti L, Remaury A, Leger F, Langin D, Lafontan M, Carpéné C, Parini A (1999) High expression of monoamine oxidases in human white adipose tissue: evidence for their involvement in noradrenaline clearance. Biochem Pharmacol 58(11):1735–1742
Qu LL, Guo NN, Li BM (2008) Beta1- and beta2-adrenoceptors in basolateral nucleus of amygdala and their roles in consolidation of fear memory in rats. Hippocampus 18:1131–1139
Sabban EL (2007) Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul 41:61–73
Sabban EL, Kvetnansky R (2001) Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci 24:91–98
Sabban EL, Liu X, Serova L, Gueorguiev V, Kvetnansky R (2006) Stress triggered changes in gene expression in adrenal medulla: transcriptional responses to acute and chronic stress. Cell Mol Neurobiol 26:845–856
Schaffler A, Buchler C (2007) Coincise review: adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells 25:818–827
Slavin BG, Ballard KW (1978) Morphological studies on the adrenergic innervation of white adipose tissue. Anat Rec 191:377–389
Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL (1994) Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol 152:3024–3031
Sudo A (1985) Accumulation of adrenaline in sympathetic nerve endings in various organs of the rat exposed to swimming stress. Jpn J Pharmacol 38:367–374
Sudo A (1987) Adrenaline in various organs of the rat: its origin, location and diurnal fluctuacion. Life Sci 41:2477–2484
Tchoukalova YD, Koutsari K, Karpyak MV, Votruba SV, Wendland E, Jensen MD (2008) Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 87:56–63
Tillinger A, Sollas A, Serova LI, Kvetnansky R, Sabban EL (2010) Vesicular monoamine transporters (VMATs) in adrenal chromaffin cells: stress-triggered induction of VMAT2 and expression in epinephrine synthesizing cells. Cell Mol Neurobiol 30:1459–1465
Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R (2011) Adipocytes as a new source of catecholamine production. FEBS Lett 585:2279–2284
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830
Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, Gimble JM (2010) Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy 12(4):538–546
Zhu XH, He QL, Lin ZH (2003) Effect of catecholamines on human preadipocyte proliferation and differentiation. Zhongua Xing Wai Ke Za Zhi 19:282–284
Acknowledgments
This research was supported by Slovak Research and Development Agency (No. APVV-0088-10 and 0148–06); TRANSMED 2, ITMS: 26240120030; VEGA Grants (2/0188/09 and 2/0036/11) and EFSD New Horizonts Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kvetnansky, R., Ukropec, J., Laukova, M. et al. Stress Stimulates Production of Catecholamines in Rat Adipocytes. Cell Mol Neurobiol 32, 801–813 (2012). https://doi.org/10.1007/s10571-012-9822-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-012-9822-6