Abstract
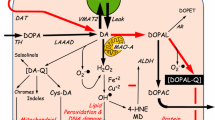
As populations age, the prevalence of geriatric neurodegenerative diseases will increase. These diseases generally are multifactorial, arising from complex interactions among genes, environment, concurrent morbidities, treatments, and time. This essay provides a concept for the pathogenesis of Lewy body diseases such as Parkinson disease, by considering them in the context of allostasis and allostatic load. Allostasis reflects active, adaptive processes that maintain apparent steady states, via multiple, interacting effectors regulated by homeostatic comparators—“homeostats.” Stress can be defined as a condition or state in which a sensed discrepancy between afferent information and a setpoint for response leads to activation of effectors, reducing the discrepancy. “Allostatic load” refers to the consequences of sustained or repeated activation of mediators of allostasis. From the analogy of an idling car, the revolutions per minute of the engine can be maintained at any of a variety of levels (allostatic states). Just as allostatic load (cumulative wear and tear) reflects design and manufacturing variations, byproducts of combustion, and time, eventually leading to engine breakdown, allostatic load in catecholaminergic neurons might eventually lead to Lewy body diseases. Central to the argument is that catecholaminergic neurons leak vesicular contents into the cytoplasm continuously during life and that catecholamines in the neuronal cytoplasm are autotoxic. These neurons therefore depend on vesicular sequestration to limit autotoxicity of cytosolic transmitter. Parkinson disease might be a disease of the elderly because of allostatic load, which depends on genetic predispositions, environmental exposures, repeated stress-related catecholamine release, and time.



Similar content being viewed by others
References
Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O’Dell M, Li SW, Pan Y, Chung HD, Galvin JE (2008) Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol 115(2):193–203
Creveling CR (2000) The role of catechol quinone species in cellular toxicity. FP Graham Publishing Co., Johnson City
Ehringer H, Hornykiewicz O (1960) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Wien Klin Wochenschr 38:1236–1239
Eisenhofer G, Kopin IJ, Goldstein DS (2004a) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56(3):331–349
Eisenhofer G, Kopin IJ, Goldstein DS (2004b) Leaky catecholamine stores: undue waste or a stress response coping mechanism? Ann N Y Acad Sci 1018:224–230
Goldstein DS (2006) Adrenaline and the inner world: an introduction to scientific integrative medicine. The Johns Hopkins University Press, Baltimore
Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC (2011) Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol 18:703–710
Goldstein DS, Li ST, Kopin IJ (2001) Sympathetic neurocirculatory failure in Parkinson disease: evidence for an etiologic role of alpha-synuclein. Ann Intern Med 135(11):1010–1011
Graboys T (2008) Life in the balance. Sterling, New York
Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA (2009) Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol 22(5):835–841
Kaufmann H, Biaggioni I (2003) Autonomic failure in neurodegenerative disorders. Semin Neurol 23(4):351–363
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849):426–430
Mosharov EV, Staal RG, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen KE, Moore CM, Troyer MD, Edwards RH, Przedborski S, Sulzer D (2006) Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci 26(36):9304–9311
Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D (2009) Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62(2):218–229
Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE (2010) The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One 5(12):e15251
Park SS, Schulz EM, Lee D (2007) Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein. Eur J Neurosci 26(11):3104–3112
Rees JN, Florang VR, Eckert LL, Doorn JA (2009) Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol 22(7):1256–1263
Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS (2008) Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson’s disease with orthostatic hypotension. Mov Disord 23(12):1725–1732
Singleton A, Gwinn-Hardy K, Sharabi Y, Li ST, Holmes C, Dendi R, Hardy J, Crawley A, Goldstein DS (2004) Association between cardiac denervation and parkinsonism caused by alpha-synuclein gene triplication. Brain 127(Pt 4):768–772
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840
Acknowledgments
Research leading to the concepts in this essay was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke. The author thanks Drs. Yehonatan Sharabi, Basil Eldadah, Graeme Eisenhofer, and Irwin J. Kopin for many valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldstein, D.S. Stress, Allostatic Load, Catecholamines, and Other Neurotransmitters in Neurodegenerative Diseases. Cell Mol Neurobiol 32, 661–666 (2012). https://doi.org/10.1007/s10571-011-9780-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9780-4