Summary
-
1.
Maple syrup urine disease (MSUD) is an inherited metabolic disorder predominantly characterized by neurological dysfunction and cerebral atrophy whose patophysiology is poorly known.
-
2.
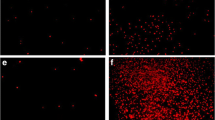
We investigated here whether the branched-chain amino acids (BCAA) leucine (Leu), isoleucine (Ile) and valine (Val), which are the biochemical hallmark of this disorder, could alter astrocyte morphology and cytoskeleton reorganization by exposing cultured astrocytes from cerebral cortex of neonatal rats to various concentrations of the amino acids. A change of cell morphology from the usual polygonal to the appearence of fusiform or process-bearing cells was caused by the BCAA. Cell death was also observed when astrocytes were incubated in the presence of BCAA for longer periods.
-
3.
Val-treated astrocytes presented the most dramatic morphological alterations. Immunocytochemistry with anti-actin and anti-GFAP antibodies revealed that all BCAA induced reorganization of actin and GFAP cytoskeleton. In addition, lysophosphatidic acid, an activator of RhoA GTPase pathway, was able to totally prevent the morphological alterations and cytoskeletal reorganization induced by Val, indicating that the RhoA signaling pathway was involved in these effects.
-
4.
Furthermore, creatine attenuated the morphological alterations provoked by the BCAA, the protection being more pronounced for Val, suggesting that impairment of energy homeostasis is partially involved in BCAA cytotoxic action. The data indicate that the BCAA accumulating in MSUD are toxic to astrocyte cells, a fact that may be related to the pathogenesis of the neurological dysfunction of MSUD patients.
Similar content being viewed by others
References
Aschner, M. (2000). Neuron-astrocyte interactions: Implications for cellular energetics and antioxidant levels. Neurotoxicology 21:1101–1107.
Appel, S. H. (1966). Inhibition of brain protein synthesis: an approach to a biochemical basis of neurological dysfunction in the amino-acidurias. Trans. N. Y. Acad. Sci. 29:63–70.
Araujo, P., Wassermann, G. F., Tallini, K., Furlanetto, V., Vargas, C. R., Wannmacher, C. M., and Wajner, M. (2001). Reduction of large neutral amino acid level in plasma and brain of hyperleucinemic rats. Neurochem. Int. 38:529–537.
Barile, M. F., Hopps, H. E., and Grabowski, M. (1978). Incidence and sources of mycoplasma contamination: a brief review. In McGarrity, G. J., Murphy, D. G., and Nichols, W. W. (eds.), Mycoplasma Infection of Cell Cultures, Plenum Press, New York, pp. 35–46.
Bignami, A., and Dahl, D. (1976). The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific protein (GFAP) in mammalian and submammalian vertebrates. Neuropathol. Appl. Neurobiol. 2:99–111.
Chuang, D. T., and Shih, V. E. (2001). Maple syrup urine disease (branched-chain ketoaciduria). In Scriver, C. R., Beaudet, A. L., Sly, W. L., and Valle. D. (eds.), The Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill, New York, pp. 1971–2005.
Cimarosti, H., Rodnight, R., Tavares, A., Paiva, R., Valentim, L., Rocha E., and Salbego, C. (2001). An investigation of the neuroprotective effect of lithium in organotypic slice cultures of rat hippocampus exposed to oxygen and glucose deprivation. Neurosci. Lett. 315:33–36.
Cotrina, M. L., Lin J. H., and Nedergaard, M. (1998). Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J. Neurosci. 18:8794–8804.
Danbolt, N. C. (2001). Glutamate uptake. Progr. Neurobiol. 65:1–105.
Danner, D. J., and Elsas, J. L. (1989). Disorders of branched chain amino acid and keto acid metabolism. In Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D. (eds). The Metabolic Basis of Inherited Disease. Mc Graw Hill, New York, pp. 671–692.
Dent, E. W., and Gestler, F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40:209–227.
Duan, S., Anderson, C. M., Stein B. A., and Swanson, R. A. (1999). Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J. Neurosci. 19:10193–10200.
Efron, M. L. (1965). Aminoaciduria. N. Engl. J. Med. 272:1058–1067.
Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420:629–635.
Fukushima, N., Kimura, Y., and Chun, J. (1998). A single receptor encoded by vzg-l/IpAl/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc. Natl. Acad. Sci. U.S.A. 95:6151–6156.
Funchal, C., de Lima Pelaez, P., Oliveira Loureiro, S., Vivian, L., Dall Bello Pessutto, F., Almeida, L. M. V., Wofchuk, S. T., Wajner, M., and Pessoa-Pureur, R. (2002). α-Ketoisocaproic acid regulates phosphorylation of intermediate filaments in postnatal rat cortical slices through ionotropic glutamatergic receptors. Dev. Brain Res. 139:267–276.
Funchal, C., Gottfried, C., Almeida, L. M. V., Wajner, M., and Pessoa-Pureur, R. (2004). Evidence that the branched chain α-keto acids accumulating in maple syrup urine disease induce morphological alterations and death in cultured astrocytes from rat cerebral cortex. Glia 48:230–240.
George, E. B., Schneider, B. F., Lasek R. J., and Katz, M. J. (1988). Axonal shortening and the mechanisms of axonal motility. Cell Mot. Cytoskel. 9:48–59.
Gottfried, C., Valentim, L., Salbego, C., Karl, J., Wofchuk, S. T., and Rodnight, R. (1999). Regulation of protein phosphorylation in astrocyte cultures by external calcium ions: Specific effects on the phosphorylation of GFAP, vimentin and heat shock protein 27 (HSP 27). Brain Res. 833:142–149.
Guasch, R. M., Tomas, M., Miñambres, R., Valles, S., Renau-Piqueras, J., and Guerri, C. (2003). Rho A and lysophosphatidic acid are involved in the actin cytoskeleton reorganization of astrocytes exposed to ethanol. J. Neurosci. Res. 72:487–502.
Halestrap, A., Brand M. D., and Denton, R. M. (1974). Inhibition of mitochondrial pyruvate transport by phenylpyruvate and α-ketoisocaproate. Biochem. Biophys. Acta. 367:102–108.
Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279:509–514.
Helfand, B. T., Chang, L., and Goldman, R. D. (2004). Intermediate filaments are dynamic and motile elements of cellular architecture. J. Cell Sci. 117:133–141.
Howell, R. K., and Lee, M. (1963). Influence of alpha-ketoacids on the respiration of brain in vitro. Proc. Soc. Exp. Biol. Med. 113:660–663.
Jouvet, P., Rustin, P., Taylor, D. L., Pocock, J. M., Felderhoff-Mueser, U., Mazarakis, N. D., Sarrat, C., Joashi, U., Kozma, M., Greewood, K., Edwards A. D., and Mehmet, H. (2000). Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: Implications for neurological impairment associated with maple syrup urine disease. Mol. Biol. Cell 11:1919–1932.
Kindy, M. S., Bhat, A. N., and Bhat, N. R. (1992). Transient ischemia stimulate glial fibrillary acidic protein and vimentin gene expression in the gerbil neocortex, striatum and hippocampus. Mol. Brain Res. 13:199–206.
Lamigeon, C., Bellier, J. P., Sacchettoni, S., Rujano, M., and Jacquemont, B. (2001). Enhanced neuronal protection from oxidative stress by coculture with glutamic acid decarboxylase-expression astrocytes. J. Neurochem. 77:598–606.
Land, J. M., Mowbray, J., and Clark, J. B. (1976). Control of pyruvate and β-hydroxy-butyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J. Neurochem. 26:823–830.
Lascola, C. D., Nelson, D. J., and Kraig, R. P. (1998). Cytoskeletal actin gates a Cl− channel in neocortical astrocytes. J. Neurosci. 18:1679–1692.
Luo, L. (2002). Actin cytoskeleton regulation in neuronal morpho genesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18:601–635.
Mackay, D. J. G., and Hall, A. (1998). Rho GTPases. J. Biol. Chem. 273:20685–20688.
Manning, T. J., Rosenfeld, S. S., and Sontheimer, H. (1998). Lysophosphatidic acid stimulates actomyosin contraction in astrocytes. J. Neurosci. Res. 53:343–352.
Nedergaard, M., Ranson, B., and Goldman, S. A. (2003). New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 26:523–530.
Nobes, C. D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62.
Nyhan, W. L. (1984). Abnormalities in Amino Acid Metabolism in Clinical Medicine, Appleton-Century-Crofts, Norwalk.
Pilla, C., Cardozo, R. F., Dutra-Filho, C. S., Wyse, A. T., Wajner, M., and Wannmacher, C. M. (2003). Creatine kinase activity from rat brain is inhibited by branched-chain amino acids in vitro. Neurochem. Res. 28:675–679.
Pringle, A., Benhan, C., Sim, L., Kennedy, J., Iannoti, F., and Sundstrom, L. (1996). Selective N-type calcium channel antagonist omega-conotoxin MVIIA is neuroprotective against hypoxic neurodegeneration in organotypic hippocampal slices cultures. Stroke 27:2124–2130.
Ridley, A. J., and Hall, A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399.
Ridley, A. J., and Hall, A. (1994). Signal transduction pathways regulating Rho-mediated stress fiber formation: Requirement for tyrosine kinase. EMBO J. 13:2600–2610.
Rosenblatt, J., Raff, M. C., and Cramer, L. P. (2001). An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11:1847–1857.
Schmidt, A., and Hall, M. N. (1998). Signaling to the actin cytoskeleton. Annu. Ver. Cell Dev. Biol. 14:305–338.
Sener, R. N. (2002). Diffusion magnetic resonance imaging in intermediate form of maple syrup urine disease. J. Neuroimaging 12:368–370.
Sergeeva, M. M., Ubl, J. J., and Reiser, G. (2000). Disruption of actin cytoskeleton in cultured rat astrocytes suppresses ATP- and bradykinin-induced (Ca2+)I oscillations by reducing the coupling efficiency between Ca2+ release, capacitative Ca2+ entry, and sore refilling, Neuroscience 97:765–769.
Sgaravati, A. M., Rosa, R. B., Schuck, P. F. Ribeiro, C. A. J., Wannmacher, C. M. D., Wyse, A. T. S., Dutra-Filho, C. S, and Wajner, M. (2003). Inhibition of brain energy metabolism by the α-keto acids accumulating in maple syrup urine disease. Biochim. Biophys. Acta. 1639:232–238.
Snyderman, E., Norton, P. M., and Roitman, B. (1964). Maple syrup urine disease, with particular reference to dietotherapy. Pediatrics 34:454–472.
Steinert, P. M., and Roop, D. R. (1988). Molecular and cellular biology of intermediate filaments. Annu. Ver. Biochem. 57:593–625.
Suidan, H. S., Nobes, C. D., Hall, A., and Monard, D. (1997). Astrocyte spreading in response to thrombin and lysophosphatidic acid is dependent on the Rho GTPase. Glia 21:244–252.
Taketomi, T., Kunishita, T., Hara, A., and Mizushima, S. (1983). Abnormal protein and lipidic composition of the cerebral myelin of a patient with maple syrup urine disease. Jpn. J. Exp. Med. 53:109–116.
Tashian, R. (1961). Inhibition of brain glutamic acid descarboxylase by phenylalanine, leucine and valine derivates: A suggestion concerning the neurological defect in phenylketonuria and branched-chain keto aciduria. Metabolism 10:393–400.
Treacy, E., Clow, C. L., Reade, T. R., Chitayat, D., Mamer, O. A., and Scriver, C. R. (1992). Maple syrup urine disease: Interrelations between branched-chain amino-, oxo- and hydroxyacids; implications for treatment; associations with CNS dysmyelination. J. Inherit. Metab. Dis. 15:121–135.
Tribble, D., and Shapira, R. (1983). Myelin proteins: Degradation in rat brain initiated by metabolites causative of maple syrup urine disease. Biochem. Biophys. Res. Commun. 114:440–446.
Uziel, G., Savoiardo, M., and Nardocci. N. (1988). CT and MRI in maple syrup urine disease. Neurology 38:486–488.
Vijayan, V. K., Goddes, J. W., Anderson, K. J., Chang-Chui, H., Ellis, W. G., and Cotman, C.W. (1991). Astrocytic hypertrophy in the Alzheimer’s disease hippocampal formation. Exp. Neurol. 112:72–79.
Wajner, M., and Vargas, C. R. (1999). Reduction of plasma concentrations of large neutral amino acids in patients with maple urine disease during crises. Arch. Dis. Child 80:579.
Wajner, M., Coelho, D. M., Barschak, A. G., Araújo, P. R., Pires, R. F., Lulhier, F. L. G., and Vargas, C. R. (2000). Reduction of large neutral amino acid concentration in plasma and CSF of patients with maple syrup urine disease during crises. J. Inher. Met. Dis. 23:505–512.
Yudkoff, M. (1997). Brain metabolism of branched-chain amino acids. Glia 21:92–98.
Zigmond, S. H. (1996). Signal transduction and actin filament organization. Curr. Opin. Cell Biol. 8:66–73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Funchal, C., Gottfried, C., Almeida, L.M.V.d. et al. Morphological Alterations and Cell Death Provoked by the Branched-Chain α-Amino Acids Accumulating in Maple Syrup Urine Disease in Astrocytes from Rat Cerebral Cortex. Cell Mol Neurobiol 25, 851–867 (2005). https://doi.org/10.1007/s10571-005-4938-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10571-005-4938-6