Abstract
The anionic nature of both cellulose fibres and reactive dyes prevents substantial exhaustion of dye from the dyebath, which is at neutral pH before alkali is added to initiate dye fixation. Conventionally, salt is added to minimize the electrostatic repulsions that interfere with dye sorption, but that increases salt loads in effluents. An alternative is to affix cationic agents on the cellulose to overcome the inherent anionic charge, but that has generally been observed to result in uneven dye sorption. The focus of investigations in this work is to examine the influence of the ratio of charges on cellulose (of affixed cationic charges to inherent anionic charges) on the extents and evenness of dye sorption. The cationisation agent 3-chloro-2-hydroxypropyl-N,N,N-trimethylammonium chloride (CHPTAC) was grafted on loose viscose fibres to yield 12 to 185 mmol kg−1 cationic group content on the fibre that exhibited an inherent carboxyl group content of 68 mmol kg−1. Three different dyes (of varying molecular sizes and anionic group content) were employed for examination of sorption profiles. The results from both zeta potential measurements and dye sorption profiles showed evidence of limited dye uptake until the cationic group content in fibres exceeded that of the inherent carboxyl groups. Thereafter, an uptick in dye sorption was observed, with dye sorption levels increasing with rise in degree of cationisation. There were differences between the dyes in their degrees of sorption, which appear correlated with their molecular sizes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactive dyeing represents the most important technology for the colouration of cellulose textiles, including cotton, viscose and lyocell (Burkinshaw and Salihu 2019a). The process of dyeing comprises two stages: first, dye liquor in neutral pH range (5–7) is circulated through substrates to ensure uniform distribution and sorption of the colorant, and then alkali is added (sodium carbonate or sodium hydroxide) to catalyse dye fixation through covalent bond formation with cellulosic hydroxy groups (Lewis 2014). One challenge in reactive dyeing is the question of how dye uptake can be promoted in the sorption stage of the process. The origin of the problem lies in the anionic nature of both reactive dyes and cellulose fibres, which results in electrostatic repulsion between dye and fibre. The dye structure is composed of a chromophoric group, which contains sulphonate groups to promote solubilisation, and a reactive group that forms a covalent linkage with the substrate (Lewis 2014). Cellulose fibres are characterised by the presence of hydroxy groups as integral part of the polymer structure and carboxyl groups, which arise from oxidative processes (Burkinshaw and Salihu 2019a). In a neutral aqueous solution, fibres develop an anionic charge due to deprotonation of carboxyl groups (pKa 3–4 (Fujisawa et al. 2011)) and at a sufficient alkaline pH hydroxy groups are partly deprotonated (pKa 12–13.5 (Bialik et al. 2016)).
In conventional dyeing processes, sorption of dyes is enhanced through the addition of large amounts of inorganic electrolytes to the dyebaths (sodium chloride or sodium sulphate at 10–100 g L−1). The promotional effect is attributed to charge screening and/or reduction in dye solubilisation (Burkinshaw and Salihu 2019b). Following the dyeing process, extensive rinsing is necessary to remove unfixed dye molecules, resulting in the production of large volumes of coloured wastewater containing high loads of inorganic electrolytes (Burkinshaw and Salihu 2018). In order to improve the ecological profile of reactive dyeing operations, it is essential to minimise the quantity of effluents and contaminants discharged.
One approach for achieving this is reverse micelle dyeing, where the dye is suspended in a non-aqueous phase using dispersants (Fu C et al. 2015; Tang and Kan 2020; Tang et al. 2018). Due to the dye’s high affinity for a thin aqueous liquid film on the fibre and the resulting high concentration gradient, it is possible to achieve high levels of dye penetration into the fibre and fixation. The removal and recovery of organic phases after dyeing is associated with a high energy cost. Another approach is the use of cationic reactive dyes, which allow salt free reactive dyeing with high exhaustion and fixation rates (Xiao et al. 2017). While electrostatic attraction between dye and fibre significantly improves dye uptake, cationic dyes are associated with disadvantages of low solubility and inadequate light fastness (Wang et al. 2020).
Yet another approach, instead of altering the dyeing medium or dye chemistry, is to modify the cellulose structure (i.e., derivatisation) by introducing cationic groups (Li et al. 2022; Tang et al. 2021; Yu et al. 2021). This strategy, known as cationisation, permits the use of existing reactive dyes and represents the most widely explored alternative route in reactive dyeing over the last 30 years (Lewis and Lei 1991). Although the benefits of cationisation and salt free reactive dyeing has been demonstrated at laboratory and pilot-scale levels, and positive assessments have been made of the economic potential and eco-friendliness of this approach, it is still not widely practiced at industrial scale (Farrell and Hauser 2013; Nallathambi and Venkateshwarapuram Rengaswami 2016; Zhai S et al. 2022). In a report published by the U.S. non-profit trade association Cotton Incorporated, two main reasons are cited for low uptake of this technology. One is the low efficiency of cationisation reactions. The second is that existing dyeing recipes and protocols do not yield the same results on cationised cellulose, and thus they need to be re-adapted which generates additional costs (Cotton Incorporated 2018).
It is crucial to recognise that the transition from conventional to salt free reactive dyeing of cationised cellulose is not a straightforward process of merely replacing the salt load with a cationisation pre-treatment. The change significantly alters the dyeing properties of the fibre. For instance, when sorption isotherms of cationic fibres are recorded, Type I, or Langmuir type isotherms are observed, in contrast to Freundlich isotherms that are typical of conventional sorption processes (Zhai X et al. 2022). Furthermore, the outcome of reactive dyeing is known to be influenced by the choice of the cationisation agent, the cationisation level and the percentage of dye exhaustion, with results varying from dye to dye (Correia et al. 2021; Pruś et al. 2023). Thus, a more fundamental understanding of the sorption mechanism and the interaction between reactive dyes and cationic modified fibres may contribute to greater ease and predictability in adaptations of existing dyeing recipes.
From a molecular perspective, cationised cellulose fibres represent a bifunctional fibre containing anionic (carboxyl) and grafted cationic (ammonium) groups. The carboxyl group content varies between 15 and 60 mmol kg−1, depending on the fibre type and history (Fitz-Binder and Bechtold 2012; Fras et al. 2004; Zemljič et al. 2008). Typical cationisation levels reported in literature far exceed the fibre carboxyl group content and start around 100 mmol kg−1, which generally results in high rates of dye sorption but uneven distribution of the dye on fibres (Fu S et al. 2016, 2017; Wu et al. 2022). However, recent studies have indicated that cationisation levels below 80 mmol kg−1 may be beneficial for salt free reactive dyeing (Setthayanond et al. 2023). At these levels, the cationic group content is similar to the carboxyl group content. Given that anionic dyes exhibit electrostatic attraction to cationic groups and repulsion to carboxyl groups, it is essential to understand the interplay between those groups in the sorption stage of reactive dyeing. Such studies are lacking, yet highly pertinent to understand the sorption mechanism.
Furthermore, as stated above, sorption profiles of reactive dyes on cationic fibres follows a Langmuir isotherm, indicating a plateau in dye sorption levels due to the presence of limited sorption sites. While it is clear that for a given dye, the plateau levels in sorption will change with the degree of cationisation, as dye uptake rises with increasing cationisation levels (Setthayanond et al. 2023; Zhang T et al. 2021), it is as yet unclear what causes differences of plateau levels between different dyes at the same degree of cationisation (Hashem et al. 2006). The molecular properties of the dye, such as the size and number of anionic groups, may have influence on the sorption properties but are rarely considered in interpretations of sorption behaviour (Clipson and Roberts 1989, 1994; Papapetros et al. 2023).
The aim of this study was to gain insights into how the molecular properties of dyes influence their sorption on cationised cellulose. Viscose fibres were cationised with 3-chloro-2-hydroxypropyl-N,N,N-trimethylammonium chloride (CHPTAC) in order to achieve a series of relative proportions between inherent carboxyl and grafted cationic groups. CHPTAC was selected as the cationisation agent due to its small molecular size to maximise the uniformity of its distribution through the fibres. In particular CHPTAC was selected over the active epoxide 2-epoxypropyl-N,N,N-trimethylammonium chloride (EPTAC) as it is more stable and is easily converted in the active compound EPTAC under alkaline conditions. The fibres were characterised by means of nitrogen analysis, FTIR spectroscopy and zeta potential measurements. The sorption properties were investigated with three dyes, differing in the number of anionic groups and molecular size. The geometric aspects of the cationisation agent and the dyes were studied by density functional theory (DFT) calculations.
Experimental
2.1. Materials and chemicals
Viscose fibres (Danufil® F) of 38 mm length and linear density of 1.7 dtex were kindly donated by Kelheim Fibres GmbH, Germany. The inherent carboxyl group content of fibres from the same batch was determined to 67.5 ± 0.4 mmol kg−1 by back-titration (Bogner et al. 2024). The reagent 3-chloro-2-hydroxypropyl-N,N,N-trimethylammonium chloride (CHPTAC) was used as cationisation agent. C.I. Acid Blue 25 (AB25), C.I. Reactive Blue 19 (RB19) and C.I. Reactive Red 120 (RR120) were obtained from Sigma-Aldrich as mono-, di- or hexasodium salts, respectively, and were used to investigate the sorption behaviour with three different dyes. L-Aspartic Acid (ASP, Mw = 133.1 g mol−1, HPLC-grade, N-content = 10.52 wt%) was used for calibration of the Nitrogen Analyser. All other reagents, HCl, sodium acetate, NaOH, KCl, KOH, acetic acid, dimethylformamide (DMF), NaCl, were at minimum, of reagent grade. The agar was of microbiology grade (Carl Roth). Physicochemical information on the cationisation agent and the dyes are available in Table 1.
Acid Blue 25 is an acid dye and is commonly used to dye fibres carrying a cationic charge, such as protein or polyamide fibres (Ghodbane and Hamdaoui 2010). Reactive Blue 19 and Reactive Red 120 are commonly used dyes for cellulose and cationised cellulose (Wang L et al. 2009). All three dyes are anionic due to the presence of one or more sulphate/sulphonate groups. The structure of AB25 and RB19 is quite similar, with the exception that RB19 contains an additional substitution on the aniline functionality of a [2-(sulphooxy)ethyl] sulphonyl group. The selected dyes vary in their molecular sizes and content of anionic groups. While AB25 carries one sulphonate group and therefore one anionic charge, RB19 contains one sulphonate and one sulphate group, resulting in twofold anionic charge. In contrast, RR120 has a molecular weight that is two or three times that of RB19 or AB25 and carries six sulphonate groups, which correspond to six anionic charges. All sorption measurements were performed in the neutral range of pH (5–7), which mitigates any hydrolysis of the vinyl sulfone anchor and thus no changes are expected in the number of anionic charges per dye molecule during sorption.
2.2. Computational analysis
Geometry optimisations were explored using quantum mechanics methods (DFT, B3LYP, 6–31 + G(d)) employing the Gaussian 16 software (Frisch et al. 2016). Visualisation of the electron density maps was performed with the GaussView 6 software (Dennington et al. 2019). The dimensions of the optimised geometries were obtained using the mulitwfn software (Lu and Chen 2012). The molecular volume \(V\) was derived from the space occupied by the electron density greater than 0.01 electrons Bohr−1 and the dimension of the molecule \(a,b\) and \(c\) were estimated from the size of the smallest box enclosing the system.
2.3. Fibre treatments
Demineralisation: In an initial treatment, to remove any residual spin finish and minerals, the fibres were immersed and agitated in an aqueous solution of HCl (0.5 wt%) at a material to liquor ratio (M:L) of 1 g: 35 mL and agitated in a shaking water bath at 40 °C for 60 min. The fibres were then removed, rinsed with deionised water, and immersed in a solution of sodium acetate (1 g L−1) with M:L 1 g: 35 mL at 40 °C for 30 min for neutralisation of any residual acid. The fibres were then rinsed again with deionised water, dried in air, and separated into loose fibres by passing through a hand-operated drum card (Giant, Woolmakers, Netherlands).
Cationisation: The demineralised fibres were cationised in an exhaust procedure at M:L 1 g: 20 mL. About 20 g of loose fibre bundle was first immersed in 350 mL aqueous solution of the cationisation agent CHPTAC in closed bottles, and agitated on a roller shaker for 30 min to ensure uniform wetting and even distribution of cationisation agent through the bundle. Then 50 mL of aqueous NaOH was added, and the bottles further agitated on the roller shaker for 30 min. The bottles were then transferred to a shaking water bath, where the temperature was raised within 30 min from ambient to 70 °C, and maintained at that temperature for 60 min. Thereafter, the fibres were separated from the reaction mixture, immersed in acetic acid solution (1 wt%) with M:L 1 g: 20 mL at 70 °C for 30 min, then rinsed twice in 1 wt% acetic acid at ambient temperature, and then rinsed several times with deionised water until the pH of the wash liquor was similar to that of the deionised water (pH of 5.5–6.5). Whereas the molar ratio NaOH:CHPTAC was maintained at 1.8: 1 in all treatments, the molar ratio CHPTAC: AGU was varied between 0.05 and 0.3: 1. In total, twelve different cationisation treatments were performed, in triplicate. See table S1 for more details.
2.4. Analysis of cationised cellulose fibres
Nitrogen analysis: Nitrogen analysis was performed by the thermal combustion Dumas method on a rapid N III Nitrogen analyser (Elementar Analysensysteme GmbH, Germany). To remove N-containing residues, fibres were subjected to an acidic washing step and gloves were worn when handling fibres to avoid transfer of impurities such as amino acids. A carefully weighed mass of about 300 mg fibres was packed in tin foil and pressed into a tablet on a hand-operated press. To remove entrapped air, the formed tablets were covered with agar gel (1.5 wt%) at 95 °C, placed in tubes, and centrifuged at 4000 rpm for 10 min. After, the agar was allowed to solidify, any excess was shaved off, and the samples loaded on the testing device for measurement. It was previously determined that the agar exhibited no significant nitrogen content. This sample preparation allowed to lower the background value and to obtain results with good reproducibility and low error (further details in supplementary). The untreated reference exhibited a nitrogen content of 0.019 ± 0.003% wt, which was the background value and deducted from the sample measurements. Five specimens per cationisation treatment were measured, and the sensitivity of the instrument in the working range was demonstrated using L-asparagine acid as standard substance, which gave a linear calibration curve (Figure S2).
Nitrogen content and the degree of substitution (DS) are calculated according to Eq. 1 and 2. \(N_{total}^{ + }\) is the nitrogen content (mmol kg−1), \(N\%\) the percentual nitrogen weight fraction (%), \(A_{r} \left( N \right)\) the atomic weight of nitrogen (14.0067 g mol−1), \(M_{w} \left( {AGU} \right)\) the molecular mass of an anhydroglucose unit (162.15 g mol−1) and \(M_{w} \left( {HPTA} \right)\) the molecular weight of the substituted 2-hydroxypropyltrimethyl(N,N,N)ammonium chloride (151.64 g mol−1).
Zeta potential measurements: The electrokinetic properties of fibres were determined with the streaming potential method, on a SurPASS 3 device (Anton Paar GmbH, Austria) using a cylindrical measurement cell. The streaming medium was composed of 1 mM KCl, and ca. 1 mM KOH was added to adjust the pH to ca. 10–11. About 0.5 g fibre specimens were pre-soaked in streaming media for 30 min before they were packed into the measurement cell, where the packing density was adjusted with aid of the instrument software to a nominal permeability index value of about 120. The zeta potential was recorded as the pH was incrementally adjusted from the initial value of 10–11 to 2, in steps of 0.3 units, with addition of HCl (0.1 mol L−1). Three specimens per cationisation treatment were measured and zeta-potential vs pH scans are shown as the average of these three measurements.
ATR-FTIR spectroscopy: ATR-FTIR spectra of cationised and untreated reference fibres were recorded on an Invenio S spectrometer (Bruker Optik GmbH, Germany), equipped with a MIRacle™ Horizontal ATR accessory fitted with a diamond crystal double reflection universal plate from PIKE Technologies (USA). The absorbance spectra were recorded from an aggregate of 128 scans in the wavenumber range of 4000–500 cm−1 at a resolution of 2 cm−1. Three measurements were performed per cationisation treatment.
Dye sorption measurements: The sorption profiles of three different dyes (AB25, RB19, RR120) were measured from their individual solutions on three specimens each from the cationised and non-cationised (reference) fibres. A carefully weighed mass of 0.5 g fibres was immersed in solution containing only the dyestuff dissolved in deionised water (0.15 g L−1 or 0.25 g L−1) with M:L 1 g: 400 mL, at 30 °C for 24 h in a shaking water bath. With dye concentrations of 0.15 g L−1, a near complete exhaustion of the dye in the solution was observed in measurements with fibre samples exhibiting the highest level of cationisation. For such fibres, therefore the initial dye concentration in solutions was adjusted to 0.25 g L−1. It was necessary to ensure that the pH of the dye solution was neutral to avoid protonation of carboxyl groups or fixation of the dye on the fibre. Both could affect the sorption equilibria. The pH of the dye solution was found to be in the neutral range between 5 and 7. After, the fibres were separated from the solutions and rinsed with deionized water until the wash-off was colourless. The residual dye content in the dye solution was determined with UV–VIS photometry on a single beam spectrophotometer (ZEISS MCS 600, equipped with MCS601 UV-NIR C spectrometer and CLH600 lamp 360–1015 nm), from the absorbance at their respective wavelengths of maximum absorbance (602 nm for AB25, 595 nm for RB19, 535 nm for RR120), with aid of linear calibration curves constructed with the same dyestuffs.
It was necessary to confirm absence of any dye fixation, which would interfere with the sorption equilibria. To that end, 50 mg portions from dyed specimens were extracted for 30 min at 100 °C in 10 mL of 50/50 mixtures by volume of DMF with water that contained 50 g L−1 NaCl. In all cases the fibres were rendered almost colourless, although in some cases, more than one cycle of extraction was required for the purpose.
Colour measurements: The reflectance curve of samples across the range 400 − 700 nm was recorded, with specular component excluded, on a d/8 spectrophotometer equipped with a pulsed Xenon lamp as light source (Model CM 3610d, Konica Minolta, Japan). Five measurements were performed per each of the three dyed specimens, each on a measurement area equivalent to 8 mm diameter. The colour strength was determined according to the Kubelka–Munk equation at one wavelength, where \(K\) is the absorption coefficient, \(S\) is the scattering coefficient and \(R_{\infty }\) is the fractional reflectance (Eq. 3).
Results and discussion
3.1. Computational analysis
The results of the geometry optimisation are presented in Table 1. The cationisation agent (CHPTAC) and the reaction intermediate (EPTAC) are relatively small agents, with the longest dimension being approximately 1 nm. The longest dimension of the dye molecules ranges from 1.54 to 3.72 nm. Electrostatic potential maps illustrate the electrostatic potential as a function of the electron density, where red, green and blue indicate areas with negative, neutral and positive electrostatic potential, respectively.
3.2. Reaction mechanism
Figure 1 illustrates a reaction scheme of the cationisation of cellulose with CHPTAC and a mechanism for the sorption state in conventional and salt-free reactive dyeing. Under neutral and mildly acidic conditions, CHPTAC is a stable precursor and is easier and safer to handle than EPTAC. However, under alkaline conditions, EPTAC is generated in situ from CHPTAC (step a). An ionised hydroxy group from cellulose can act as a nucleophilic centre and attack the epoxide functionality of EPTAC, initiating the ring-opening reaction and leading to covalent grafting of the cationisation agent (step b). Substitution of (2-hydroxypropyl)trimethylammonium can occur on the C2, C3 and C6 hydroxy groups. In the case of a small residue, such as CHPTAC, grafting is observed on all three hydroxy groups, with C6 and C2 being equally favoured compared to C3 (order of preference: C6 ≈ C2 > C3) (de la Motte et al. 2011; Etale et al. 2021; Gupta et al. 2023). The role of NaOH is multifaceted, as it can either promote or inhibit the reaction (Prado and Matulewicz 2014). One equivalent of NaOH is consumed to generate EPTAC from CHPTAC, while another is required for the deprotonation of a hydroxy group, thereby creating a nucleophilic centre. As NaOH itself is already a strong nucleophile, direct hydrolysis of EPTAC to (2,3-dihydroxypropyl)trimethylammonium chloride (DHPTAC) occurs as a side reaction (step b).
3.3. Degree of cationisation
The cationisation level was determined from the nitrogen content of the cationised fibre. A linear correlation was observed between the nitrogen content and the CHPTAC concentration in the treatment solution (Fig. 2).
The nitrogen content as function of the CHPTAC concentration during cationisation treatment. The cationic samples are divided into three distinct zones. In zone I, the fibre exhibits a significantly lower cationic group content than carboxyl group content. In zone II, the cationic and carboxyl group contents are similar. In zone III, the cationic group content is significantly higher than the carboxyl group content
as function of the CHPTAC concentration during cationisation treatment. The cationic samples are divided into three distinct zones. In zone I, the fibre exhibits a significantly lower cationic group content than carboxyl group content. In zone II, the cationic and carboxyl group contents are similar. In zone III, the cationic group content is significantly higher than the carboxyl group content
The nitrogen content of the modified fibres was determined to be between 0.0170 and 0.259 wt%
(12–185 mmol kg−1), corresponding to a degree of substitution (DS) of 0.002–0.03. The low concentration of CHPTAC in the cationisation solution results in a cationisation levels that are relatively low compared to the DS of 0.05–0.5 and above, as reported in the literature (Hashem and El-Shishtawy 2001; Liu et al. 2022; Pi-xin et al. 2009; Wang et al. 2009; Zhang et al. 2016). Approximately 10% of the CHPTAC is grafted onto the fibre, with the remaining CHPTAC lost due to hydrolysis in solution, which is well documented in the literature (Hashem and El-Shishtawy 2001). In this study no further attempts were made to enhance the overall performance of the cationisation.
Cationisation with CHPTAC introduces one cationic group to the fibre through the grafting of one quaternary ammonium group. The nitrogen content is equivalent to the cationic group content. For the sake of clarity and comprehension, the nitrogen group content will be referred to as the cationic group content throughout the text.
3.4. ATR-FTIR spectroscopy
Figure 3 shows the FTIR spectra of an untreated and representative cationised fibres selected from zone I, II and III. The most prominent peaks for the untreated viscose fibre occur at 3640–3000, 2917, 2850, 1150, 1020 and 895 cm−1 and are assigned to OH stretching, CH stretching, CH2 stretching, C–O–C asymmetric stretching, C–O stretching and group C1 frequency, respectively. A peak at 1640 cm−1 is attributed to O–H stretching of adsorbed water on cellulose (Carrillo et al. 2004). Since the viscose fibres have a carboxyl group content of 68 mmol kg−1, a peak from the carboxyl group is expected to occur at a wavenumber of 1600 cm−1 (Bogner et al. 2024). Nevertheless, due to the relatively low amount of carboxyl groups and the overlapping flank of the 1640 cm−1 peak, it is not possible to observe a distinct carboxyl peak.
Derivatisation with a cationisation agent results in the formation of new C–O–C bonds and the introduction of additional C–H, O–H and C–N bonds. In FTIR spectroscopy, the C–O–C stretching vibration overlaps with the C–O–C vibrations of the β-glycosidic linkage and the O–H vibration overlaps with the hydroxy groups of cellulose. For these reasons, the successful grafting of the molecule is commonly indicated by the emergence of an absorption band at the wavenumber of 1480 cm−1. This peak is characteristic of the quaternary ammonium group and is attributed to the bending mode of the C–H bonds from the methyl groups (Čížová et al. 2016; Etale et al. 2021; Sajomsang et al. 2009; Sekhavat Pour et al. 2015; Zhang et al. 2016). In the recorded spectra, no peak can be assigned to 1480 cm−1. However, a shoulder of the peak is assigned to 1465 cm−1. The C–H vibration overlaps the viscose background, which explains why only the flanks of this peak are visible and the shoulder occurs at lower wavenumbers. The absence of a distinctive IR-peak is attributed to the low degree of substitution with a cationic group content that is significantly below 1%wt.
3.5. Zeta potential
The zeta or \(\zeta\)-potential represents the electrokinetic state in the shear plane, which is influenced by the functional groups of the fibre and the electrolyte (Luxbacher 2020). The change in the \(\zeta\)-potential, can be explained by the accumulation of the dominant molecules and ions in the electrolyte. The adsorption of water and, due to its amphoteric character hydronium and hydroxide ions on the surface of the fibre, together with their subsequent penetration into the fibre influence the surface charge and the \(\zeta\)-potential (Luxbacher 2020).
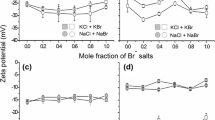
Figure 4a shows the \(\zeta\)-potential profile of the untreated viscose fibre, which exhibits the typical shape of cellulosic fibres (Luxbacher 2014). Over a wide range, a negative \(\zeta\)-potential is observed as a result of dissociated carboxyl groups. At a pH value below 4.5 to 5, protonation of the carboxylate begins and as the COOH: COO− ratio increases, the \(\zeta\)-potential rises and reaches positive values at pH values below 2.1. The pH value at which the \(\zeta\)-potential curve crosses the zero potential line is the isoelectric point (IEP). This value is in good agreement with literature values for viscose (Ristić et al. 2015). A slight hump is observed in the pH range of 10 to 5, which is attributed to the swelling and deswelling of the fibre (Stana-Kleinschek et al. 2001).
The zeta potential profiles as a function of pH for the untreated reference fibre  and the cationised fibres from zones I, II and III are presented in figure a. The cationic group content (mmol kg−1) of the modified fibres is given in the legend. The pH of IEP
and the cationised fibres from zones I, II and III are presented in figure a. The cationic group content (mmol kg−1) of the modified fibres is given in the legend. The pH of IEP  and \(\left( {\Delta \zeta /\Delta pH} \right)_{IEP}\)
and \(\left( {\Delta \zeta /\Delta pH} \right)_{IEP}\) as a function of the cationic group content exhibits a linear correlation throughout all zones (figure b). For \(\left( {\Delta \zeta /\Delta pH} \right)_{IEP}\) two linear sections are observed, one from zone I to II and the other from zone III
as a function of the cationic group content exhibits a linear correlation throughout all zones (figure b). For \(\left( {\Delta \zeta /\Delta pH} \right)_{IEP}\) two linear sections are observed, one from zone I to II and the other from zone III
The cationised fibres can be divided into three zones based on the shape of the \(\zeta\)-potential profile (Fig. 4a). Fibres with a degree of cationisation below 35 mmol kg−1 (zone I) exhibit \(\zeta\)-potential curves that are similar to the untreated reference. Fibres with a cationic group content above 92 mmol kg−1 (zone III) exhibit straightened \(\zeta\)-potential profiles, in which significant features, such as the hump between pH 10 to 5 and the strong increase in the zeta potential below pH 4.5, are absent. A maximum near pH 7.5 is observed, which is most probably due to swelling effects. In zone II, fibres have a cationic group content of 48–83 mmol kg−1, with the corresponding \(\zeta\)-potential profiles situated between those of zone I and III. As the cationic group content increases beyond 68 mmol kg−1, the shape of the \(\zeta\)-potential profile undergoes a transition from a profile resembling that of zone I to one resembling that of zone III.
All cationised fibres exhibit a common property: the pH of the isoelectric point (IEP) is shifted in a neutral direction with a good linear correlation to the cationisation level (Fig. 4b). Another characteristic is the value \(\left( {{\Delta }\zeta /{\Delta }pH} \right)_{IEP}\), which can be derived from the slope of the curve at the point where the zero potential line is crossed. Zone I exhibits a higher \(\left( {{\Delta }\zeta /{\Delta }pH} \right)_{IEP}\) value in comparison to the untreated reference, yet as the cationisation level increases, \(\left( {{\Delta }\zeta /{\Delta }p} \right)_{IEP}\) decreases. By analysing \(\left( {{\Delta }\zeta /{\Delta }p} \right)_{IEP}\) as a function of the cationisation level, two sections can be identified. The first section includes all fibres from zone I and II. Here, the protonation of carboxyl groups contributes to the change in the \(\zeta\)-potential at the pH where the zero potential line is crossed. Consequently, \(\left( {\Delta \zeta /\Delta p} \right)_{IEP}\) is significantly higher (pH of IEP < 4.5). Within this row, a decrease of \(\left( {\Delta \zeta /\Delta p} \right)_{IEP}\) can be observed with increasing cationisation levels, as the contributing effect from the protonation becomes less significant. The second section comprises fibres from zone III, which exhibit a pH of IEP exceeding 4.5. At this pH value, there is minimal protonation of carboxyl groups, and the zero potential line is crossed without this contribution, resulting in significantly lower \(\left( {\Delta \zeta /\Delta pH} \right)_{IEP}\) values.
One possible explanation for this phenomenon is that the contribution of carboxyl groups to the \(\zeta\)-potential is compensated and overruled with increasing numbers of cationic groups. This can be observed in the change in the \(\zeta\)-potential profile within zone II as the amount of cationic groups exceeds the amount of carboxyl groups (transition of cationic group content from 64 to 71 mmol kg−1). In fibres with a high cationic group content, these groups determine the shape of the \(\zeta\)-potential curves and mask any contribution from carboxyl groups and the swelling-deswelling of the fibres (Tarbuk et al. 2014). When the number of cationic groups exceeds that of anionic groups, the fibre’s net charge becomes positive, but the zeta potential remains negative. It is important to note that the zeta potential represents the state at the shear plane and does not represent the fibre surface charge. The dominant ions present in the shear plane are influenced by the fibre’s functional groups, as well as its hydrophilicity and wettability. It is known that cationised fibres exhibit higher hydrophilicity and wettability compared to their untreated counterparts (Park and Choi 2018; Wu et al. 2022). Increasing hydrophilicity results in a less negative \(\zeta\)-potentials (Kuehn et al. 1986; Ribitsch et al. 2001). The alteration of negative to positive \(\zeta\)-potentials for samples of zone II and III cannot be attributed to the protonation of carboxyl groups. Instead, it is due to a change in the accumulation of dominant ion species in the shear plane. The determination of the \(\zeta\)-potential is influenced by a multitude of factors, including the fibre surface, hydrophilicity, wettability, electrolyte composition, pH, packing density, and others as discussed in the IUPAC Technical Report (Delgado et al. 2005). Consequently it is not possible to attribute the observed phenomena to a single underlying mechanism.
3.6. Sorption experiments
Figure 5 shows the dye uptake as a function of the cationic group content of the modified fibres. Similar trends in sorption behaviour are observed for all three dye structures. At low cationisation levels, strong repulsive forces between anionic groups from fibre and dye need to be overcome and result in little to no dye uptake.
The dye uptake of AB25  , RB19
, RB19  and RR120
and RR120  is presented as a function of the cationic group content. The dye uptake exhibits a similar pattern for all dyes. In zones I and II, the slope of the dye uptake is relatively low, but a significant increase is observed at cationisation levels above the carboxyl group content
is presented as a function of the cationic group content. The dye uptake exhibits a similar pattern for all dyes. In zones I and II, the slope of the dye uptake is relatively low, but a significant increase is observed at cationisation levels above the carboxyl group content
(< 1 mmol kg−1). As the content of cationic groups increases from zone I to II the repulsive forces of the carboxyl groups are progressively neutralised by cationic groups, resulting in a significant increase in dye uptake for AB25 and RB19, and to a lesser extent for RR120.
Within zone II, the slope of the dye uptake vs cationic group content curve changes. The change in slope is observed at the intersection points of the two linear sections assigned to data points from zone I to II and zone II to III. This change occurs at a cationic group content of 56 ± 4 mmol kg−1, which is sufficient to neutralise the repulsive force from the carboxyl groups, but lower than the actual carboxyl group content determined by back-titration (68 mmol kg−1). It is important to note that both methods, the back titration and the Dumas analysis, determine the total carboxyl group or total nitrogen content of the fibre, respectively. Therefore, this discrepancy may be explained by the different distribution of carboxyl and cationic groups. Viscose fibres are spun from a solution and precipitated in a coagulation bath, resulting in the distribution of carboxyl groups throughout the cross-section (Bogner et al. 2024). In contrast, cationisation is a fibre pre-treatment that is more likely to occur on the accessible surface and pores of the fibre. At all cationisation levels the dominant sorption mechanism is attributed to ionic interactions between dye molecules and accessible cationic groups. Cationic groups may act as localised sorption sites, enabling anionic dye molecules to adsorb in proximity to the cationic group via an ion exchange mechanism. This may explain the sorption observed on anionic surfaces, as the total charge for some fibres (i.e. fibres from zone I) and the zeta potential for all fibres is negative in the pH region (5–7), where the sorption experiments were performed. In addition to ionic interactions, non-ionic and non-covalent interactions contribute to the sorption of dye molecules. However, these interactions are less dominant and are believed to contribute to the total binding force and reorientation of the adsorbed dye molecule.
The absence of dye fixation was confirmed by dye extraction experiments, which yielded mostly colourless samples (Figures S4, S5 and S6). For RR120, complete removal of adsorbed dye was not possible for all cationisation levels, even after several rounds of dye extraction. However, spectroscopic measurements confirmed that 90% of the dye was removed after the first round of dye extraction.
3.7. Colour photometry
The results of the colour photometric measurements are presented in the form of K/Smax values as a function of the dye content on the fibre. A linear correlation is observed (Fig. 6). For AB25 and RR120, the K/S curves begin to approach a plateau at 25 and 10 mmol kg−1 dye content, respectively. This is due to the technical limitations of the measurement method and device, as the reflectance falls below 2% for K/S values above 25, making it difficult to distinguish between the different samples.
The earlier approach of the plateau and the difference in the slope of the curve can be explained by the absorption coefficient \(K\), which depends on the dye structure. The molar extinction coefficients, calculated from the calibration graphs in accordance with the Beer-Lambert law (Bechtold and Pham 2023) are 13,200 ± 400, 26,900 ± 700 and 58,400 ± 800 L mol−1 cm−1 for RB19, AB25 and RR120, respectively. The ratio between the individual molar extinction coefficients corresponds to the ratio between the slopes of the linear part of the K/S vs dye uptake curves. In the case of RB19, a linear response of the K/S values was obtained over the whole range of dye uptake, as this dye has the lowest molar extinction coefficient.
3.8. Visual impression of the dyed fibres
Figure 7 illustrates the optical appearance of fibres from zones I, II and III. The lowest cationisation level (12 mmol kg−1) exhibits no dye uptake and appears colourless. An increase in the cationisation level to 34 mmol kg−1 enables dye uptake on the fibre, although the sample exhibits uneven dyeing, with the presence of both coloured and uncoloured areas.
It can be observed that successive increases in the cationisation level result in more uniform dyeing (zone II, III). This is exemplified by the cationisation levels of 80 and 185 mmol kg−1. A slight variation in colour and shade is a consequence of the random arrangement of loose fibres within the bundle, which results in non-uniform light reflections and the creation of shadows. This phenomenon is most clearly observed in undyed fibres, thus indicating that it is not a result of unevenness in dye uptake. Further photographs can be found in the supplementary material (Figures S4, S5 and S6).
3.9. Discussion and interpretation of the cationisation and sorption mechanism
Zeta potential measurements and sorption studies permit an understanding of the interaction between cellulose’s carboxyl groups and grafted cationic groups. From the zeta potential measurements, it can be observed that there is a distinctive change in the shape of the zeta potential when the cationic group content compensates for the carboxyl group content. In the sorption profiles, dye uptake increases significantly as the carboxyl groups are sufficiently neutralised by the cationic groups. This neutralisation may be attributed to the formation of ionic bonds between carboxyl groups and cationic groups, which necessitates the close proximity of both groups. It is believed that cationisation occurs randomly on the fibre, as CHPTAC and EPTAC do not have a strong affinity for cellulose fibres. This is reflected in the low cationisation yield. However, the presence of a quaternary ammonium functionality makes the cationisation agent susceptible to electrostatic attraction by carboxyl groups. It is therefore reasonable to assume that at low cationisation levels, CHPTAC adsorbs in proximity to carboxyl groups and is covalently grafted with the addition of alkali to surrounding hydroxy groups. Nevertheless, no direct evidence is presented in this work to substantiate this assumption.
The observation of Langmuir isotherms for cationised fibres and reactive dyes indicates that there exists a limited number of sorption sites. The limitation in dye uptake and the stagnation in the plateau are a consequence of the complete coverage of the available sorption sites. The number of sorption sites is dependent on the cellulose fibre, specifically the degree of cationisation and the adsorbent, which is defined by the size and number of anionic groups present in the dye. If the number of anionic groups or the size of the dye molecule is the determining factor in dye sorption, a linear correlation between dye uptake and one of these two properties should exist. Consequently, the dimensions of the molecule in a, b, and c, as well as the number of anionic groups, are plotted against the dye uptake for a specific cationisation level.
Figure 8 shows the existence of a linear correlation between the dimension of the second longest side and the dye uptake. This observation demonstrates that the approach of the plateau is not solely a function of the cationsiation level and makes it necessary to consider the molecular structure of the dye especially the size of the dye molecule. This phenomenon is observed for all investigated cationisation levels. With regard to the pore size and pore size distribution in viscose fibres, this observation becomes reasonable, as the dimensions of the dyes are similar to the size of the pores (Bredereck et al. 1985; Fras et al. 2004; Zemljic et al. 2012).
Consequently, we propose the following interrelationship between the level of cationisation, dye uptake and carboxyl group content: The cationisation with a small cationisation agents, such as CHPTAC and EPTAC occurs randomly on the fibre with good accessibility to pores, but for low cationisation levels, adsorption and fixation of the cationisation agents occurs in proximity to carboxyl groups. The size of the dye molecule and the accessibility to cationic groups and to pores is the limiting factor in dye sorption. Moreover, it appears that the second longest dimension demonstrates the strongest correlation with regard to dye uptake, as dye molecules may orientate themselves to have optimal accessibility to cellulose’s slit pores. Furthermore, especially at low levels of cationisation, the distance between two cationic groups is too great for a single dye molecule to cover more than one cationic groups simultaneously. In a nutshell, this can be deduced from the inner surface area of the fibre, which is approximately 440 m2g−1 as determined by inverse size exclusion chromatography (ISEC) (Bredereck et al. 1985; Fras et al. 2004; Zemljic et al. 2012), and the cationic group content. Consequently, the adsorption of a dye molecule by a cationic group may not be dependent on the number of anionic groups present.
Conclusion
The objective of this study was to investigate the dye sorption behaviour of low-level cationised viscose fibres with a cationic group content of 12–185 mmol kg−1, which is within the range of the carboxyl group content (68 mmol kg−1) of processed cellulose fibres. The results of the study contribute to the understanding of the fundamental principles of cellulose cationisation and dyeing of cationic cellulose fibres, with particular emphasis on the sorption stage of reactive dyeing. It is demonstrated that cationisation must compensate for a distinct amount of carboxyl groups in order to promote dye uptake. At cationisation levels below the carboxyl group content, the shape of the zeta potential and the sorption behaviour are determined by the presence of carboxyl groups. At cationisation levels approximating the carboxyl group content, a significant change in the shape of the zeta potential and a notable increase in dye sorption are observed. The observations are consistent with the hypothesis that increased dye uptake can be expected when the degree of cationisation exceeds the carboxyl group content. Furthermore, the molecular structure of the dye, particularly its size, explains the different plateau values for dye uptake. This emphasises the necessity of considering the pore structure of the fibre and the size of the dye molecules. For instance, the cationising agents EPTAC and CHPTAC are relatively small molecules and show good accessibility to small pores that are inaccessible to dye molecules.
These observations reveal the importance of considering the inherent carboxyl group content of the fibre as well as the molecular structure of dyes and their accessibility to the fibre. Further investigations in this direction, i.e., inverse size exclusion chromatography or similar studies on the accessibility of molecules to the pores of the fibre, are part of future work. Furthermore, the second stage of reactive dyeing, fixation, requires further investigation to ascertain the significance of the sorption mechanism for the fixation stage. An understanding of the transition from a reversible dyeing system without fixation to a non-reversible dyeing system with dye fixation may facilitate the development of more feasible salt free dyeing routes and the readaptation of reactive dyeing recipes.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Bechtold T, Pham T (2023) Basics of colour development. In: Textile Chemistry, De Gruyter, Berlin, Boston https://doi.org/10.1515/9783110795738
Bialik E, Stenqvist B, Fang Y et al (2016) Ionization of cellobiose in aqueous alkali and the mechanism of cellulose dissolution. J Phys Chem Lett 7:5044–5048. https://doi.org/10.1021/acs.jpclett.6b02346
Bogner P, Bechtold T, Pham T, Manian AP (2024) Alkali induced changes in spatial distribution of functional groups in carboxymethylated cellulose. Cellulose 31:2833–2847. https://doi.org/10.1007/s10570-024-05798-9
Bredereck K, Schick WA, Bader E (1985) Zur bestimmung der porenstruktur wassergequollener cellulosefasern. Makromol Chem 186:1643–1655. https://doi.org/10.1002/macp.1985.021860812
Burkinshaw SM, Salihu G (2018) The role of auxiliaries in the immersion dyeing of textile fibres: part 10 the influence of inorganic electrolyte on the wash-off of reactive dyes. Dyes Pigment 149:652–661. https://doi.org/10.1016/j.dyepig.2017.11.034
Burkinshaw SM, Salihu G (2019a) The role of auxiliaries in the immersion dyeing of textile fibres part 2: analysis of conventional models that describe the manner by which inorganic electrolytes promote direct dye uptake on cellulosic fibres. Dyes Pigment 161:531–545. https://doi.org/10.1016/j.dyepig.2017.08.034
Burkinshaw SM, Salihu G (2019b) The role of auxiliaries in the immersion dyeing of textile fibres: part 7 theoretical models to describe the mechanism by which inorganic electrolytes promote reactive dye uptake on cellulosic fibres. Dyes Pigment 161:605–613. https://doi.org/10.1016/j.dyepig.2017.09.024
Carrillo F, Colom X, Suñol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur Polym J 40:2229–2234. https://doi.org/10.1016/j.eurpolymj.2004.05.003
Čížová A, Neščáková Z, Malovíková A, Bystrický S (2016) Preparation and characterization of cationic and amphoteric mannans from Candida albicans. Carbohydr Polym 149:1–7. https://doi.org/10.1016/j.carbpol.2016.04.083
Clipson JA, Roberts GAF (1989) Differential dyeing cotton. 1—Preparation and evaluation of differential dyeing cotton yarn. J Soc Dyers Colour 105:158–162. https://doi.org/10.1111/j.1478-4408.1989.tb01204.x
Clipson JA, Roberts GAF (1994) Differential dyeing cotton. Part 2-stoichiometry of interaction with acid and direct dyes. J Soc Dyers Colour 110:69–73. https://doi.org/10.1111/j.1478-4408.1994.tb01611.x
Correia J, Oliveira FR, Rd CSCV, Valle JAB (2021) Preparation of cationic cotton through reaction with different polyelectrolytes. Cellulose 28:1–22. https://doi.org/10.1007/s10570-021-04260-4
de la Motte H, Hasani M, Brelid H, Westman G (2011) Molecular characterization of hydrolyzed cationized nanocrystalline cellulose, cotton cellulose and softwood kraft pulp using high resolution 1D and 2D NMR. Carbohydr Polym 85:738–746. https://doi.org/10.1016/j.carbpol.2011.03.038
Delgado AV, González-Caballero F, Hunter RJ, Koopal LK, Lyklema J (2005) Measurement and interpretation of electrokinetic phenomena (IUPAC technical report). Pure Appl Chem 77:1753–1805. https://doi.org/10.1351/pac200577101753
Dennington R, Keith TA, Millam JM. (2019). GaussView Version 6. Semichem Inc., Schawnee Mission, KS.
Etale A, Nhlane DS, Mosai AK et al (2021) Synthesis and application of cationised cellulose for removal of Cr(VI) from acid mine-drainage contaminated water. AAS Open Res. https://doi.org/10.12688/aasopenres.13182.1
Farrell M, Hauser P (2013) Cationic cotton, reservations to reality. AATCC Rev, 13:56. https://www.researchgate.net/publication/274382080
Fitz-Binder C, Bechtold T (2012) Ca2+ sorption on regenerated cellulose fibres. Carbohydr Polym 90:937–942. https://doi.org/10.1016/j.Carbpol.2012.06.023
Fras L, Laine J, Stenius P et al (2004) Determination of dissociable groups in natural and regenerated cellulose fibers by different titration methods. J Appl Polym Sci 92:3186–3195. https://doi.org/10.1002/app.20294
Frisch MJ, Trucks GW, Schlegel HB, et al (2016). Gaussian 16 Rev. C.01. Wallingford, CT.
Fu C, Wang J, Shao J et al (2015) A non-aqueous dyeing process of reactive dye on cotton. J Text I 106:152–161. https://doi.org/10.1080/00405000.2014.906103
Fu S, Farrell MJ, Hauser PJ et al (2016) Real-time dyebath monitoring of reactive dyeing on cationized cotton for levelness control: part 1—influence of dye structure, temperature, and addition of soda ash. Cellulose 23:3319–3330. https://doi.org/10.1007/s10570-016-1008-9
Fu S, Farrell MJ, Hauser PJ et al (2017) Real-time dyebath monitoring of reactive dyeing on cationized cotton for levelness control: part 2—effects of leveling agents and dye dosing. Cellulose 24:3061–3071. https://doi.org/10.1007/s10570-017-1291-0
Fujisawa S, Okita Y, Fukuzumi H, Saito T, Isogai A (2011) Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr Polym 84:579–583. https://doi.org/10.1016/j.carbpol.2010.12.029
Ghodbane H, Hamdaoui O (2010) Decolorization of antraquinonic dye, C.I. Acid Blue 25, in aqueous solution by direct UV irradiation, UV/H2O2 and UV/Fe(II) processes. Chem Eng J 160:226–231. https://doi.org/10.1016/j.cej.2010.03.049
Gupta A, Ladino CR, Mekonnen TH (2023) Cationic modification of cellulose as a sustainable and recyclable adsorbent for anionic dyes. Int J Biol Macromol 234:123523. https://doi.org/10.1016/j.ijbiomac.2023.123523
Hashem A, El-Shishtawy RM (2001) Preparation and characterization of cationized cellulose for the removal of anionic dyes. Adsorpt Sci Technol 19:197–210. https://doi.org/10.1260/0263617011494088
Hashem A, Aly AA, Aly AS (2006) Preparation and utilization of cationized sawdust. Polym-Plast Technol Eng 45:395–401. https://doi.org/10.1080/03602550600553697
Cotton Incorporated (2018) A world of ideas: Technologies for sustainable cotton textile manufactoring - volume II. Cary. https://www.cottonworks.com/wp-content/uploads/2018/09/World-of-Ideas-2_9-17-2018.pdf. Accessed 07 May 2024
Kuehn N, Jacobasch H-J, Lunkenheimer K (1986) Zum Zusammenhang zwischen dem Kontaktwinkel zwischen Wasser und festen Polymeren und ihrem Zeta-Potential gegenüber wäßrigen Lösungen. Acta Polym 37:394–396. https://doi.org/10.1002/actp.1986.010370617
Lewis DM (2014) Developments in the chemistry of reactive dyes and their application processes. Color Technol 130:382–412. https://doi.org/10.1111/cote.12114
Lewis DM, Lei XP (1991) New methods for improving the dyeability of cellulose fibres with reactive dyes. J Soc Dyers Colour 107:102–109. https://doi.org/10.1111/j.1478-4408.1991.tb01312.x
Li Y, Zhai S, Dong W et al (2022) Preparation of cationic viscose and its salt-free dyeing using reactive dye. Color Technol 138:378–387. https://doi.org/10.1111/cote.12598
Liu J, Yang R, Wang Y, Hua F, Tong S (2022) Cationic cellulose nanofibers with efficient anionic dye adsorption: adsorption mechanism and application in salt-free dyeing of paper. Cellulose 29:2047–2061. https://doi.org/10.1007/s10570-021-04406-4
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Luxbacher T (2014) The zeta guide principles of the streaming potential technique. Anton Paar GmbH, Graz
Luxbacher T (2020) 9. In: Electrokinetic properties of natural fibres, Woodhead Publishing, https://doi.org/10.1016/B978-0-12-818782-1.00009-2
Nallathambi A, Venkateshwarapuram Rengaswami GD (2016) Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr Polym 152:1–11. https://doi.org/10.1016/j.carbpol.2016.06.087
Papapetros K, Sygellou L, Anastasopoulos C et al (2023) Spectroscopic study of the interaction of reactive dyes with polymeric cationic modifiers of cotton fabrics. Appl Sci 13:5530. https://doi.org/10.3390/app13095530
Park S, Choi HM (2018) Microwave-mediated rapid oxidation and cationization of cotton cellulose. Cellul Chem Technol 52:311
Pi-xin W, Xiu-li W, Xue D-h et al (2009) Preparation and characterization of cationic corn starch with a high degree of substitution in dioxane–THF–water media. Carbohydr Res 344:851–855. https://doi.org/10.1016/j.carres.2009.02.023
Prado HJ, Matulewicz MC (2014) Cationization of polysaccharides: a path to greener derivatives with many industrial applications. Eur Polym J 52:53–75. https://doi.org/10.1016/j.eurpolymj.2013.12.011
Pruś S, Kulpiński P, Matyjas-Zgondek E, Rutowicz J, Wojciechowski K (2023) The light fastness of the reactive dyes on cationized cellulose. J Nat Fibers 20:2215995. https://doi.org/10.1080/15440478.2023.2215995
Ribitsch V, Stana-Kleinschek K, Kreze T, Strnad S (2001) The significance of surface charge and structure on the accessibility of cellulose fibres. Macromol Mater Eng 286:648–654. https://doi.org/10.1002/1439-2054(20011001)286:10%3c648::aid-mame648%3e3.0.co;2-6
Ristić T, Hribernik S, Fras-Zemljič L (2015) Electrokinetic properties of fibres functionalised by chitosan and chitosan nanoparticles. Cellulose 22:3811–3823. https://doi.org/10.1007/s10570-015-0760-6
Sajomsang W, Gonil P, Tantayanon S (2009) Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: preparation and characterization. Int J Biol Macromol 44:419–427. https://doi.org/10.1016/j.ijbiomac.2009.03.003
Sekhavat Pour Z, Makvandi P, Ghaemy M (2015) Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly(vinyl alcohol). Int J Biol Macromol 80:596–604. https://doi.org/10.1016/j.ijbiomac.2015.07.008
Setthayanond J, Netzer F, Seemork K et al (2023) Low-level cationisation of cotton opens a chemical saving route to salt free reactive dyeing. Cellulose 30:4697–4711. https://doi.org/10.1007/s10570-023-05136-5
Stana-Kleinschek K, Kreze T, Ribitsch V, Strnad S (2001) Reactivity and electrokinetical properties of different types of regenerated cellulose fibres. Colloids Surf A Physicochem Eng Asp 195:275–284. https://doi.org/10.1016/s0927-7757(01)00852-4
Tang AYL, Kan CW (2020) Non-aqueous dyeing of cotton fibre with reactive dyes: a review. Color Technol 136:214–223. https://doi.org/10.1111/cote.12459
Tang AYL, Lee CH, Wang Y, Kan CW (2018) Dyeing properties of cotton with reactive dye in nonane nonaqueous reverse micelle system. ACS Omega 3:2812–2819. https://doi.org/10.1021/acsomega.8b00032
Tang P, Lockett L-ME, Zhang M, Sun G (2021) Modification of cotton fabrics with 2-diethylaminoethyl chloride for salt-free dyeing with anionic dyes. Cellulose 28:6699–6712. https://doi.org/10.1007/s10570-021-03942-3
Tarbuk A, Grancaric AM, Leskovac M (2014) Novel cotton cellulose by cationisation during the mercerisation process—part 1: chemical and morphological changes. Cellulose 21:2167–2179. https://doi.org/10.1007/s10570-014-0245-z
Wang L, Ma W, Zhang S, Teng X, Yang J (2009) Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr Polym 78:602–608. https://doi.org/10.1016/j.carbpol.2009.05.022
Wang M, Guo C, Li C, Zhao T (2020) Design of novel reactive dyes containing cationic groups: Mechanism and application for environmentally friendly cotton dyeing. Fiber Polym 21:2848–2860. https://doi.org/10.1007/s12221-020-1059-2
Wu A, Ma W, Yang Z, Zhang S (2022) Efficient cationization of cotton for salt-free dyeing by adjusting fiber crystallinity through alcohol-water-NaOH pretreatment. Polymers 14:5546. https://doi.org/10.3390/polym14245546
Xiao H, Zhao T, Li C-H, Li M-Y (2017) Eco-friendly approaches for dyeing multiple type of fabrics with cationic reactive dyes. J Clean pro 165:1499–1507. https://doi.org/10.1016/j.jclepro.2017.07.174
Yu C, Liu Y, Lu Y, Tao K, Yi Z (2021) A salt-free pad-irradiate-pad-steam reactive dyeing process for cotton fabric and the influence of cationising conditions on its coloration. Color Technol 137:399–406. https://doi.org/10.1111/cote.12537
Zemljič LF, Peršin Z, Stenius P (2008) Kleinschek KS (2008) Carboxyl groups in pre-treated regenerated cellulose fibres. Cellulose 15:681–690. https://doi.org/10.1007/s10570-008-9216-6
Zemljic LF, Hribernik S, Manian AP et al (2012) In: Polysaccharide fibres in textiles. Springer, Vienna
Zhai S, Li Y, Dong W et al (2022a) Cationic cotton modified by 3-chloro-2-hydroxypropyl trimethyl ammonium chloride for salt-free dyeing with high levelling performance. Cellulose 29:633–646. https://doi.org/10.1007/s10570-021-04295-7
Zhai X, Ma J, Wu Y et al (2022b) Investigation on dyeing mechanism of modified cotton fiber. RSC Adv 12:31596–31607. https://doi.org/10.1039/d2ra05668b
Zhang H, Guo H, Wang B et al (2016) Homogeneous synthesis and characterization of polyacrylamide-grafted cationic cellulose flocculants. J Appl Polym Sci 133:43106. https://doi.org/10.1002/app.43106
Zhang T, Zhang S, Qian W, He J, Dong X (2021) Reactive dyeing of cationized cotton fabric: the effect of cationization level. ACS Sustain Chem Eng 9:12355–12364. https://doi.org/10.1021/acssuschemeng.1c04340
Acknowledgments
Financial support is gratefully acknowledged to the COMET Project “Textile Competence Center Vorarlberg 2 – FFG 882502”, funded within COMET – Competence Centers for Excellent Technologies – by BMK, BMDW as well as co-financing federal province Vorarlberg. The COMET-Funding Program is managed by the Austrian Research Promotion Agency FFG.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. Open access funding provided by University of Innsbruck and Medical University of Innsbruck. The research leading to these results was performed in the frame of the project “Textile Competence Center Vorarlberg 2 (Project No. 882502)” funded under the COMET program—Competence Centers for Excellent Technologies—by the Federal Ministry for Climate Protection, Environment, Energy, Mobility, Innovation and Technology (BMK) and the Federal Ministry for Digitization and Business Location (BMDW), with co-financing from the federal province of Vorarlberg. The COMET program is administered by the Austrian Research Promotion Agency (FFG).
Author information
Authors and Affiliations
Contributions
F.N. Investigation, Methodology, Visualization, Writing—Original Draft, Review & Editing; A.P.M. Conceptualization, Methodology, Writing—Review & Editing; T.B. Supervision, Conceptualization, Methodology, Writing—Review & Editing; T.P. Funding acquisition, Supervision, Conceptualization, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable, because this article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Netzer, F., Manian, A.P., Bechtold, T. et al. The role of carboxyl and cationic groups in low-level cationised cellulose fibres investigated by zeta potential and sorption studies. Cellulose (2024). https://doi.org/10.1007/s10570-024-06132-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10570-024-06132-z






 , and of cationised fibres from zone I
, and of cationised fibres from zone I  , II
, II  and III
and III  . A full FTIR spectrum is shown in a) and b) shows a zoomed-in area for the range 1650–1150 cm−1. The cationic group content of the fibre is given in parentheses
. A full FTIR spectrum is shown in a) and b) shows a zoomed-in area for the range 1650–1150 cm−1. The cationic group content of the fibre is given in parentheses


 and RR120
and RR120  a flattening of the curve is observed at K/S values higher than 25. In the case of RB19
a flattening of the curve is observed at K/S values higher than 25. In the case of RB19  a linear correlation is observed over the whole range of dye uptake
a linear correlation is observed over the whole range of dye uptake

 , b
, b  and c
and c  of the dye molecules is presented in a) for cationisation levels of 48
of the dye molecules is presented in a) for cationisation levels of 48  and 185
and 185  mmol kg−1. Figure b) illustrates the correlation between dye uptake and the number of anionic groups of the dye for the two cationisation levels
mmol kg−1. Figure b) illustrates the correlation between dye uptake and the number of anionic groups of the dye for the two cationisation levels