Abstract
Hemicellulose-rich pulp raw materials are avoided in the production of man-made cellulosic textile fibres due to hemicellulose reactivity with the currently used industrial solvent systems. Incorporation of hemicelluloses in regenerated fibres could, however, increase the share of used wood biomass and thus improve the environmental footprint of regenerated fibre products. Superbase ionic liquids have shown potential in dissolving and regenerating all the major wood polymers i.e. cellulose, hemicellulose and lignin into regenerated products. In this work, regenerated fibres were spun from hemicellulose-rich softwood and eucalyptus paper-grade pulps and eucalyptus dissolving pulp using a superbase ionic liquid [mTBNH][OAc]. Before dissolution and spinning, intrinsic viscosities of the paper-grade pulps were adjusted either enzymatically or by using a mild acid-treatment to improve dope rheology for dry-jet wet spinning. In fibre spinning, hemicellulose was found to regenerate in high yield and the obtained regenerated fibres had high dry tenacities (5.3 to 15 cN/dtex). The best mechanical properties were measured from fibres with high hemicellulose content (17.3% (w/w)). Pulp pretreatment was found to be critical for achieving good mechanical properties. Acid-pretreatment, which modified both cellulose and hemicellulose, yielded regenerated fibres with better mechanical properties compared to an enzyme-pretreatment which did not alter the hemicellulose structure. Removal of hemicellulose substituents and hydrolysis of hemicellulose backbone in acid-pretreatment may be the key to improved mechanical properties in hemicellulose-containing regenerated fibres. Enzymatic peeling and imaging with a xylan-specific monoclonal antibody (CCRC-M138) suggest that hemicelluloses were enriched to the outermost layers of the regenerated fibres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uncoupling global material production from fossil carbon resources requires increased and intensified use of lignocellulosic biomass. However, the current practices exploiting e.g. wood biomass turn only a small proportion of wood into materials whereas the rest is typically burned to produce energy. Dissolving pulp which consists mostly of cellulose (alpha-Cellulose content > 90% (Sixta 2006) is currently the primary raw material used in commercial processes to produce regenerated cellulosic products such as viscose or lyocell-type textile fibres. Typically, only 30–35% of the wood can be turned into dissolving pulp (Balkissoon et al. 2022) whereas hemicellulose-rich bleached paper-grade pulp can be produced with a higher yield (40–45%) (Alén 2011a).
The use of hemicellulose-rich pulp grades in producing regenerated fibres has been avoided due to the adverse effects of hemicelluloses on the processing and quality of the final product. Paper-grade pulps also contain more metal ions bound to the pulp via anionic groups present in hemicelluloses and residual lignin (Su et al. 2011). Metal ions can influence the chemical stability of the used solvent. The major hemicelluloses in softwood and hardwood pulps, namely xylans and glucomannans, are chemically more reactive compared to cellulose due to their heterogeneous chemical structure, the lower molecular weight that enhances solubility and less-ordered organisation in the fibre wall. Challenges with hemicellulose in textile fibre processing are associated with their higher reactivity with chemicals used for carbohydrate polymer dissolution in commercialized processes. In the viscose process, the use of NaOH and CS2 leads to hemicellulose degradation products that are harmful both during the process and in the final product (Schild and Liftinger 2014). In the lyocell process using N-methylmorpholine N-oxide (NMMO), the solvent reacts in side reactions with the pulp carbohydrates, especially with carbonyl groups in hemicellulose (Rosenau et al. 2002a, b) forming oxidized by-products that affect the brightness of the final product.
Challenges in processing have prevented the use of hemicellulose-rich raw materials industrially but several small-scale studies have shown hemicellulose-rich regenerated cellulosic fibres to have promising properties. Higher hemicellulose content is shown to improve water retention, dyeability and fibrillation resistance of the regenerated fibres while deteriorating mechanical properties only a little if even at all (Zhang et al. 2008; Schild and Liftinger 2014; Chen et al. 2015a; Ma et al. 2016; Singh and Murthy 2017; Moriam et al. 2021). Schild and Liftinger (2014) prepared xylan containing viscose fibres by adding xylan to dissolving pulp-based dope after alkalisation and xanthation. In their study, up to 90% of the added xylan was regenerated in the fibres. Xylan content of 7.5% in the viscose fibres had only a small negative impact on mechanical properties and brightness whereas water uptake ability was increased (Schild and Liftinger 2014). Enzymatic peeling of the regenerated fibres showed enrichment of xylan on the surface of the fibres. In another study, similarly prepared viscose fibres containing 10% of xylan were found to have decreased fibre-fibre friction in a frictional force study compared to fibres with lower xylan content (Singh and Murthy 2017). Unlike the viscose process, small scale NMMO-based lyocell process can produce regenerated fibres from hemicellulose-rich raw materials directly without sequential dissolution of cellulose and hemicellulose (Zhang et al. 2008; Chen et al. 2015b). As previously noted, high hemicellulose content in the dope led to side reactions with NMMO seen as strong discolouration of the dope (Chen et al. 2015b).
Several ionic liquids (ILs) are found to dissolve cellulose. Already in 2010, almost a hundred ILs capable of dissolving cellulose were reviewed (Mäki-Arvela et al. 2010). The Ioncell process has shown that the superbase IL, [DBNH][OAc], is capable of producing strong (tenacities upto 5 cN/dtex) man-made cellulose fibres from dissolving pulps (Sixta et al. 2015; Michud et al. 2016). The superbase ILs are not reactive with carbohydrate hydroxyl groups or reducing ends (Sturm et al. 2023a) and therefore could enable the use of hemicellulose-rich raw materials in fibre spinning. The downside of superbase ILs is that they suffer from hydrolysis under aqueous conditions (Elsayed et al. 2020; Sturm et al. 2023a) complicating solvent recycling. Stepan et al. (2016a) demonstrated the use of hemicellulose-rich paper-grade pulp in the Ioncell-F process using superbase ionic liquid [DBNH][OAc]. Ma et al. (2016) used the same ionic liquid to prepare regenerated fibres with good mechanical properties from hemicellulose-rich copy paper after removing inorganic material. The mechanical properties of the regenerated fibres were improved by decreasing the hemicellulose content of the used paper raw material; dry tenacities of 3.8 and 4.4 cN/dtex were obtained from fine paper with 24 and 11% of hemicellulose, respectively. Recently, Sturm et al. (2023a) produced regenerated fibres with good mechanical properties from viscosity-adjusted paper-grade pulp (α-cellulose content < 90%) using [mTBDH][OAc] but studied the hydrolytic stability of the solvent during recycling instead of the effect of the high hemicellulose content on fibre properties. The recycled IL was reported to be free from hemicellulose components meaning that hemicellulose was regenerated into the produced fibres (Sturm et al. 2023a). Additionally, [DBNH][OAc] is shown to dissolve unbleached, hemicellulose and lignin-containing birch pulps (Ma et al. 2018). In the same study, regenerated fibres were obtained from pulps with varying amounts of hemicellulose and lignin, even with the highest amounts of hemicellulose (22%) and lignin (23%), the pulp was spinnable (Ma et al. 2018).
This work demonstrates the use of bleached hemicellulose-rich paper-grade pulp from eucalyptus and northern softwood in the production of regenerated textile fibres using a novel superbase ionic liquid [mTBNH][OAc]. The used IL has better hydrolytic stability and lower melting point than some of its predecessors (Elsayed et al. 2020; Martins et al. 2022; Sturm et al. 2023b). Two pulp pretreatments using sulfuric acid and endoglucanase enzyme were compared. The effect of pulp raw material and pulp pretreatment on hemicellulose regeneration and localisation in regenerated fibres was assessed. For the first time, the effect of hemicellulose on the properties of the regenerated fibres was assessed not only by its quantity but also by its chemical structure arising from different botanical origins (eucalyptus/softwood) and pretreatments.
Materials and methods
Materials
Eucalyptus dissolving pulp and bleached eucalyptus kraft pulp with 1.0% and 1.1% of lignin contents, respectively, were received as dry sheets (Altri group, Portugal). Bleached softwood kraft pulp with 1.0% of lignin was received as dry sheets from Metsä Fibre from a Finnish pulp mill. Cotton linter fibres were obtained by disintegrating Whatman No. 1 filter paper in water. Cotton linters were utilised only for comparative analysis in the confocal laser scanning microscopy to determine the sensitivity of xylan immunolabelling.
Endoglucanase-rich enzyme preparation Fibercare R was purchased from Novozymes (Bagsværd, Denmark). The preparation had an endoglucanase activity of 2760 nkat/ml on hydroxyethyl cellulose and a negligible xylanase activity of 4 nkat/ml at pH 5 (Rahikainen et al. 2020). The protein concentration of the preparation was 17.3 mg/ml and measured with a protein assay kit (DC protein assay, Bio-Rad Laboratories, Town, Counry) based on the Lowry method (Lowry et al. 1951). Before the protein assay, impurities interfering with the measurement were removed by protein precipitation with cold acetone followed by protein re-dissolution in an aqueous solution containing 2% (w/v) Na2CO3 and 0.4% (w/v) NaOH. The enzyme was dosed based on the protein concentration as mg of protein per g of dry pulp in the enzymatic pulp treatments.
Superbase ionic liquid 5-methyl-1,5,5-triaza-bicyclo-[4.3.0]non-6-enium acetate [mTBNH][OAc] received from the University of Helsinki was synthesized as described by Martins et al. (2022). The purity of the IL was > 96% and water content 0.0%.
Processing scheme
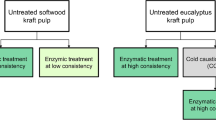
The wood pulps were processed to regenerated cellulosic fibres according to the scheme shown in Fig. 1.
Processing scheme from wood pulps to regenerated fibres. Dissolving pulp from eucalyptus (Euca-Diss) and paper-grade pulps from northern softwood (Pulp SW) and eucalyptus (Pulp Euca) were used. Pretreatments with sulfuric acid (A) and enzyme (E) were carried out for the paper-grade (kraft) pulps before pulp dissolution and spinning
Pulp pretreatments
Pulp sheets (1.5 kg) were cold disintegrated in reverse osmosis water. Shredded sheets were soaked for 2 h at 10% (w/w) consistency followed by gentle disintegration with a wet-disintegrator at 4% (w/w) consistency at 300 rpm for 15 min. Excess water was removed by filtration and the wet pulp was homogenised.
The viscosity of kraft pulps was adjusted before dissolution either by acid hydrolysis or enzymatic pretreatment. Acid hydrolysis was done at 10% (w/w) consistency using 13.5% (w/w) of H2SO4 on pulp at 90 °C for 45 min in a reactor equipped with an anchor-type mixer that mixed the slurry for 30 s at 340 rpm within 12-minute intervals. After the treatment, the pulp was washed, spin-dried to ca. 35% (w/w) dry matter content and homogenised.
The enzyme-pretreatments were performed at high pulp consistency (20% (w/w)) using an LZ05-4 laboratory mixer (Winkworth) equipped with two z-blade mixers. The treatments were carried out with 0.01 mg/g and 0.18 mg/g of endoglucanase for softwood and eucalyptus kraft pulps, respectively. The endoglucanase preparation was diluted with water and buffer (sodium phosphate pH 6) before spraying the mixture evenly on the pulp (50 g) resulting in 20% (w/w) consistency and 100 mM ionic strength in the pulp. The pulp was mixed at 50 °C 30 rpm for 2 h. After the treatment, the enzyme activity was terminated by diluting the pulp to approximately 5% (w/w) consistency with boiling water whereafter the pulp was incubated at 100 °C for 20 min followed by vacuum filtration with a 60 μm filtration cloth, washing with reverse osmosis water and homogenisation with a kitchen mixer. The treatment was repeated four times and the resulting pulp batches were combined and mixed thoroughly.
Yield losses of the pulp pretreatments were determined gravimetrically after removing solubilised carbohydrates by washing.
Dissolution of pulp samples
Ionic liquid [mTBNH][OAc] and pulp samples with dry matter contents ranging from 31.7 to 45.3% (w/w) were weighed to produce 5% (w/w) solutions. Pulp samples were mixed into ionic liquid at room temperature with an overhead mixer (Heidolph RZR 2021, Germany) for approximately 5 min. After mixing, samples were placed inside an oven (Memmert ufe 400, Germany) for preliminary dissolution of four hours at a temperature of 85 °C. After preliminary dissolution, samples were mixed and placed into an oven at 85 °C inside a custom-made vacuum reservoir for deaeration and removal of residual water for 48 h in a vacuum of approximately 1 bar. After 24 h, samples were mixed to improve the homogeneity of the solution, after which deaeration was continued for another 24 h.
The solubility of the pulp was verified before and after spinning with an optical microscope (Leitz diaplan type 020-437.035, Germany) equipped with a digital camera (Euromex TL20614, Netherlands).
Spinning of dopes
After successful deaeration, dopes of 70 g were packed into a 200 ml steel syringe (New era pump systems inc, USA), and placed into an oven to be preheated for one hour at 85 °C for improved spinnability. During spinning, dope was extruded through a spinneret with eight 300 μm holes by using a mechanical pump (Kf technology NE-8000, Italy). The coagulating bath consisted of room temperature water, through which fibres were guided with two guides, resulting in a regeneration length of 0.62 m before the collection of fibres onto a godet of custom-made laboratory-scale spinning equipment. Air-gap was set to be approximately 2 cm for improved orientation of molecules during drawing. Spinning was initiated with an input velocity of 24.8 m/min (14 ml/min) and a drawing speed of 3.8 m/min. Drawing velocity was steadily increased until a maximum velocity of 25.4 m/min was reached, after which input flow was steadily decreased to a minimum value, determined by filament breakage. Spinning was continued until depletion of the spinning dope.
After spinning, fibres collected on the godet were placed to soak in deionized water. Water was replaced three times until the discolouration of water subsided. After washing, the fibres were either dried by ethanol exchange followed by air-drying at room temperature, by freeze-drying for 24 h, or packed and sent without drying for further analyses. Ethanol exchange was conducted by placing fibres into a beaker filled with 99% ethanol. Fibres were separated from each other and left to soak for two minutes. After this, the fibres were drained from excess ethanol with a Bühner -funnel, and the fibres were dried at ambient temperature (22 ± 2 °C).
Yield losses of fibre spinning were calculated theoretically by comparing the carbohydrate compositions of the pretreated pulps and regenerated fibres with the assumption that cellulose regenerated without yield losses.
Characterization of pulp
The intrinsic viscosity of the pulp samples was determined with two parallel measurements with a maximum accepted deviation of 2% according to ISO 5351-1 using a PSL Rheotek instrument (Poulten, Selfe & Lee Ltd, UK).
Molar mass distributions (MMD) and the number of carboxyl groups in the pulp was determined by heterogeneous fluorescence labelling followed by size exclusion chromatography (SEC) according to a standard protocol (Potthast et al. 2015). For preconditioning, sample corresponding to 20 mg of dry pulp was suspended in 0.1 M HCl and agitated for 20 s in a mixer. The pulp was washed with 0.1 M HCl, ethanol 96%, and DMAc, filtered, and transferred into a 4 mL vial. For derivatization, the pulp was suspended in 3 mL of DMAc, and 1 mL of FDAM solution (approximately 0.125 mol/L in DMAc) was added. The suspension was agitated in a shaking bath at 40 °C for 7 d. The pulp was filtered off, washed with DMAc, and transferred into a dry vial. For dissolution of the cellulose, 1.6 mL of DMAc/LiCl 9% (w/v) was added. After complete dissolution with no visible residues, the sample was diluted and filtered through 0.45 μm filters and analyzed by SEC-MALLS-Fluorescence-RI.
Determination of carbohydrate composition
Carbohydrate compositions of the pulps and the regenerated fibres were determined after 2-stage sulphuric acid hydrolysis according to NREL (Sluiter et al. 2008). Lignin content was determined only for the untreated pulps. Hydrolysed monosaccharides were analysed with a high-performance anion exchange chromatography Dionex ICS-5000 and the gradient system was modified from a previously published method by Tenkanen and Siika-Aho et al. (2000) by decreasing the NaOH concentration of B gradient to 10 mM. The determined monosaccharides were calculated to correspond to polymeric weights as anhydrous saccharides. For softwood pulp, xylose and arabinose were calculated to originate from arabinoglucuronoxylan while mannose, galactose and part of glucose based on a glucose-to-mannose ratio of 1:3 (Alén 2000) were calculated to originate from galactoglucomannan and the rest of the glucose was considered to originate from cellulose. For eucalyptus pulps xylose was considered to originate from glucuronoxylan while mannose and a part of glucose based on glucose to mannose ratio 1:1.5 (Alén 2000) were considered to originate from glucomannan and the rest of the glucose was considered to originate from cellulose.
Characterization of regenerated fibres
The air-dried regenerated fibres were conditioned at a relative humidity of 65 ± 5% and temperature of 20 ± 2 °C for at least 24 h. The mechanical properties were determined as an average of 20 measurements according to ISO 1973 and ISO 5079 standards using a FAVIMAT + fibre tester (Textechno GmbH, Germany). Mechanical testing included tests for elongation (%), maximum force (cN), dry tenacity (cN/dtex), and linear density (dtex).
The crystallinity of the samples was analysed by solid-state NMR using a Bruker Avance III HD 400 spectrometer (resonance frequency of 1 H of 400.13 MHz, and 13 C of 100.61 MHz), equipped with a 4 mm dual broadband cross-polarization magic angle spinning (CP-MAS) probe. The samples were swollen overnight in deionized water before measurement. 13 C spectra were acquired with the total sideband suppression (TOSS) sequence at ambient temperature with a spinning rate of 5 kHz, a cross-polarization (CP) contact time of 2 ms, a recycle delay of 2 s, SPINAL-64 1 H decoupling and an acquisition time of 50 ms whereas the spectral width was set to 300 ppm. Chemical shifts were referenced externally against the carbonyl signal of glycine at δ = 176.03 ppm. The acquired free induction decays (FIDs) were apodized with an exponential function (lb = 11 Hz) before the Fourier transformation. Peak fitting of the C-4 signal for estimating the crystallinity index (CI) was performed with the Dmfit program (Massiot et al. 2002) according to the model of Zuckerstätter et al. (2013).
The moisture content of the regenerated fibres was determined by conditioning the oven-dried fibres in a humidity chamber set to a chosen relative humidity at the constant temperature of 25 ± 0.1 °C. The moisture content of the regenerated fibres was measured by weighting the fibres.
Characterization of hemicellulose location in the regenerated fibres
The location of hemicellulose in the regenerated fibres was estimated by enzymatic peeling and analysis of the dissolved saccharides. Enzymes are expected to catalyse the hydrolysis of the fibres from the surface towards the fibre core. Therefore, the composition of the saccharides dissolved at different hydrolysis time points gives an estimation of the hemicellulose location on the different layers of the regenerated fibres. Regenerated fibres were hydrolysed with 10 mg/g of cellulase mix containing endoglucanase (Fibercare R, 3 mg/g), cellobiohydrolase (pure T. reesei Cel7A, 6 mg/g) and β-glucosidase (pure A. niger Cel3A) and 0.5 mg/g of xylanase (pure T. reesei Xyn11A) and mannanase (pure T. reesei Man5A). The pure enzymes have been purified from the cellulase mix produced by T. reesei or from commercial enzyme preparates. Fibres were hydrolysed for 1, 4 and 24 h at 1% (w/w) consistency in 50 mM sodium acetate buffer (pH 5) at 45 °C followed by inactivating the enzymes by boiling. The samples were centrifuged and the oligosaccharides in the supernatant were hydrolysed to monosaccharides with 2.43% (w/v) sulfuric acid by autoclaving at 120 °C for 1 h. The monomeric sugars were determined using high-performance anion exchange chromatography similarly as described for the analysis of the carbohydrate composition of pulps and fibres. The monomeric sugars were calculated to correspond to polymeric weights as described above.
The morphology of the regenerated fibres and the location of xylan in the fibres were studied using confocal microscopy and antibody labelling. For the localisation of xylan, the fibre samples were immunolabelled with monoclonal, unconjugated anti-xylan antibody (CCRC-M138, Creative Diagnostics). In this procedure, solutions of the previous step were removed as a supernatant after 30 s spinning. First, aliquots of 10 mg of the samples were incubated in 1 ml of phosphate-buffered saline (PBS; Oxoid) with 0.1% (v/v) Tween-20 o/n at + 4 °C with constant mild shaking. The samples were washed by adding 1 ml of PBS with 0.1% (v/v) Tween-20 and 5% (w/v) BSA and incubating for 15 min at RT. Primary antibody CCRC-M138 diluted in 1:50 was added in 1 ml of PBS with 0.1% (v/v) Tween-20 and 5% (w/v) BSA and incubated o/n at + 4 °C with constant mild shaking. The samples were washed three times for 10–15 min at RT by adding 1 ml of PBS with 5% (w/v) BSA with constant mild shaking. Rabbit anti-mouse IgG antibody conjugated with Alexa Fluor 633 (A-21,063, Invitrogen) diluted 1:100 was added as a secondary antibody in 1 ml of PBS and incubated for o/n at + 4 °C with constant mild shaking as covered with foil. The samples were washed four times by adding 1 ml of PBS and two times by adding 1 ml of mQ-H2O for 15 min with constant mild shaking at RT covered with foil. Immunolabelled fibres were stored in mQ-H2O at + 4 °C covered with foil. Controls were immunolabelled without both primary and secondary antibodies and with secondary antibody only by substituting the omitted antibody solutions with buffer solution.
For imaging, some immunolabelled fibres were spread on a microscopy slide and stained with aqueous 0.01% (w/v) Calcofluor White (Fluorescent brightener 28, Aldrich, Germany) for the visualisation of cellulose (Fulcher et al. 1989). Before sealing the preparate, Vectashield Vibrance antifade mountant (Vector Laboratories) was added.
Detection of immunolabelling and Calcofluor White staining was done using confocal laser scanning microscopy (CLSM) equipment consisting of a Zeiss LSM 710 (Zeiss, Jena, Germany) attached to a Zeiss Axio Imager.Z microscope. A diode laser line of 405 nm was used for excitation of Calcofluor White and emission was collected at 410–490 nm. Red diode laser line 633 nm was used for excitation of secondary antibody coupled with Alexa Fluor 633, and emission was collected at 640–675 nm. For visualisation of the overall appearance of the fibre, a transmitted light detector was utilised. Images were assembled of the optical sections taken with a 20x objective (Zeiss EC Epiplan-Neofluar, numerical aperture of 0.50), a 40x objective (Zeiss EC Epiplan-Neofluar, numerical aperture of 0.75), and a 63x objective (Zeiss LCI Plan-Neofluar, numerical aperture of 1.3) and resolution of 1024 × 1024 using ZEN software (Zeiss).
The final CLSM micrographs, in which cellulose appears cyan and immunolabelled hemicellulose red, were reconstructed by superimposing two emission images and the transmission image. Representative images were selected for publication.
Results and discussion
Pulp pretreatments
The molecular weight of cellulose and hemicellulose in softwood and eucalyptus kraft pulps was lowered by chemical and enzymatic treatments before pulp dissolution and spinning (Table 1). The intrinsic viscosities of the studied softwood and eucalyptus kraft pulps were too high, 860 and 940 ml/g respectively, to enable the preparation of a dope with reasonable viscosity for spinning. The targeted intrinsic viscosity level was 420–450 ml/g which is earlier reported to be suitable for an ionic liquid-based process (Ma et al. 2018). The viscosity of softwood kraft pulp was successfully decreased close to the targeted level both by acid hydrolysis (470 ml/g) and enzyme-treatment (460 ml/g) while the viscosity of eucalyptus kraft pulp was adjusted only by enzyme-treatment (440 ml/g) (Table 1).
Molar mass distributions (MMD) of the unmodified and pretreated eucalyptus pulps were determined to evaluate how the pretreatment affected the chain lengths of cellulose and hemicellulose polymers. The MMD of the unmodified eucalyptus kraft pulp showed a bimodal distribution where the larger peak in the high molar mass area corresponds to cellulose and the smaller peak in the low molar mass area corresponds mainly to hemicellulose (Fig. 2). The MMD of the enzyme-treated eucalyptus pulp (Euca-E) was found to remain bimodal but to shift towards the low molar mass area revealing a decrease in the degree of polymerisation (DP) of cellulose. The retained strong hemicellulose “shoulder” indicates that hydrolysis of hemicellulose chains was modest in the enzyme-treatment. The MMD of the eucalyptus dissolving pulp (Euca-Diss) was located in the same area as the MMD of the Euca-E but had only a weak hemicellulose shoulder in the low molar mass area. Commonly, man-made cellulosic fibres are prepared from pulps with a narrow and unimodal distributions similar to Euca-Diss.
Measurement of the MMDs of the softwood pulp samples was unsuccessful likely due to the well know challenges related to the dissolution of softwood kraft pulp samples (Berthold et al. 2004). However, the enzyme- and acid-treatments were assumed to modify hemicelluloses to different extents. The enzyme-treatment of softwood kraft pulp was assumed to hydrolyse cellulose and leave hemicelluloses mostly intact based on the MMD of the Euca-E and the nature of the used enzyme which is shown to cleave cellulosic polymers selectively (Willberg-Keyriläinen et al. 2019). Acid-pretreatment on the other hand is unspecific and is shown to cleave both cellulosic and hemicellulosic polymers (Berggren et al. 2001). In addition, acid-treatment was found to decrease the degree of substitution of hemicellulose more than the enzyme-treatment, detected by the content of arabinose and galactose substituents, and the content of carboxylic acids corresponding to glucuronic acid substituents (Table 1). Therefore, the acid-treated pulp had less substituted and probably shorter hemicellulose chains than the enzyme-treated pulp.
Preparation and properties of the regenerated fibres
Regenerated fibres were prepared from eucalyptus dissolving pulp and pretreated eucalyptus and softwood kraft pulps. The wet pulps (32–45% (w/w) dry matter) were dissolved in ionic liquid [mTBNH][OAc] at 5% (w/w) pulp content at 85 °C and spun to regenerated fibres by small-scale dry-jet wet spinning. The spinneret used had eight holes with a diameter of 300 μm.
All studied pulps dissolved in [mTBNH][OAc] in 5% (w/w) concentration within 48 h, however, the dissolution of pulps with the highest water content proceeded slower compared to pulps with low water content since water evaporation needed to take place before pulp dissolution occurred. The presence of water often has adverse effects on the solubility of cellulosic material in ionic liquids (Hauru et al. 2012; Gericke et al., 2012). Every sample had good spinnability after dissolution; uninterrupted spinning with take-up velocity of 25.4 m/min was achieved with input velocities of 1.8, 3.5, 3.5 and 8.8 m/min for Euca-DISS, SW-A, SW-E and Euca-E respectively. A minimum input velocity of ca. 0.7 m/min could be achieved with every sample before filament breakage. Only minimal differences in the spinnabilities could be observed, except with sample Euca-E which had slightly inferior spinnability compared to the other samples.
Carbohydrate compositions of the regenerated fibres were compared to the compositions of the pulp samples (Fig. 3). In general, compositional differences between the regenerated fibres and their corresponding untreated and pretreated pulps were minor meaning that both hemicellulose and cellulose regenerated in high yield during the spinning process. Hemicellulose content in the regenerated fibres was only 1.0-1.9% points lower than in the pulps they were spun from meaning only a small decrease especially for the fibres spun from the hemicellulose-rich pulps.
Pretreatments had only a minor effect on the chemical composition of the pulps. Hemicellulose contents of the kraft pulps remained high (19.2–20.5% (w/w) after all pretreatments, thus the pulps were ideal for studying the effect of high hemicellulose content in fibre spinning. Acid- and enzyme-pretreated softwood kraft pulps had similar carbohydrate compositions (Fig. 3). Enzyme-treatment of softwood kraft pulp did not decrease the hemicellulose content which was expected due to the selective hydrolysis of cellulose with certain endoglucanases (Rahikainen et al. 2019). Acid-treatment decreased hemicellulose content only slightly (Fig. 3) even if hemicellulose is hydrolysed more easily than cellulose (Alén 2011b). Therefore, the acid- and enzyme-treated softwood kraft pulps had rather similar hemicellulose contents but the hemicellulose chains in the acid-treated pulp were probably shorter as discussed earlier. Regenerated fibres spun from acid- and enzyme-pretreated softwood pulps had similar hemicellulose contents, 17.3% (w/w) and 18.4% (w/w), respectively which suggests that even the shorter hemicellulose chains in the acid-treated pulp regenerated in high yield.
The compositional analysis was not optimal for the regenerated fibres since a higher share of regenerated fibres was not hydrolysed to monosaccharides in the sulphuric acid hydrolysis before anion exchange chromatography (Fig. 3). Therefore, it was assumed that part of the regenerated fibres was not analysed. Additionally, acidic monosaccharides (methylglucuronic acids) abundant especially in eucalyptus xylan cannot be detected after sulphuric acid hydrolysis (Shi et al. 2020).
Relative carbohydrate compositions of pulps, pretreated pulps and regenerated fibres determined after sulphuric acid hydrolysis. The abbreviations SW and Euca correspond to softwood and eucalyptus and A and E correspond to acid- and enzyme-pretreatments respectively. Error bars represent standard deviations. The yield of the acid hydrolysis is shown on top of each bar
In pulp pretreatment and fibre spinning some of the pulp carbohydrates dissolve during processing which is seen as unwanted yield loss. Yield losses of pulp pretreatments were determined gravimetrically and yield losses of fibre spinning were calculated based on the carbohydrate compositions of pulps and regenerated fibres (Table 2). Yield loss from fibre spinning was assumed to originate from the incomplete regeneration of hemicellulose while cellulose was assumed to have regenerated without yield losses (Ma et al. 2016). The total yield losses were small (1.7–3.2% (w/w) for all the regenerated fibres which maintains a high raw material efficiency compared to dissolving pulp.
The acid-pretreatment of softwood pulp resulted in a two-fold higher yield loss compared to the enzyme-treatment, 0.9 and 0.4% (w/w), respectively (Table 2), which was predicted based on the unselective nature of the acid-treatment. However, enzyme-treatment of eucalyptus kraft pulp had the highest yield loss (1.9% (w/w) (Table 2). The high yield loss may originate from the relatively high enzyme dose that was needed with eucalyptus kraft pulp to reach the targeted viscosity level. Eucalyptus kraft pulp was less prone to enzyme-treatment than softwood kraft pulp as shown in a previous study (Spönla et al. 2023). This is thought to originate mainly from the different structures and chemical compositions of eucalyptus and softwood kraft fibres (Spönla et al. 2023). This suggests that the high endoglucanase dose hydrolysed surface of the eucalyptus fibres at a higher extent even to water-soluble oligomers before the average degree of polymerisation of cellulose was decreased to the desired level. The highest yield loss in spinning was calculated for the acid-treated softwood kraft pulp. This could originate from the probably more fragmented hemicellulose chains in the acid-treated pulp that may be less likely to regenerate compared to longer hemicellulose chains.
Mechanical properties, spinning parameters, crystallinity indexes and total hemicellulose contents of the spun fibres are shown in Table 3. The average dry tenacities varied from 5.3 to 15 cN/dtex. The values are high for man-made cellulosic fibres that typically range from 2.5 cN/dtex in commercial viscose fibres to 4 cN/dtex in lyocell fibres from the NMMO process (Röder et al. 2013). The high dry tenacities may be associated with the high draw ratios that are possible to reach with the used small-scale spinning equipment. The positive effect of a high draw ratio on the orientation of cellulose chains (Azimi et al. 2022), and on mechanical properties is known (El Seoud et al. 2020). A previous study using paper-grade pulp as raw material and the same ionic liquid [mTBNH][OAc] as a solvent, reported tenacity values of 4 cN/dtex when using larger spinning equipment (Sturm et al. 2023b). High tenacities (4.8–5.8 cN/dtex) have been obtained in studies where regenerated fibres were obtained from dissolving pulps and [DBNH][OAc] as a solvent (Sixta et al. 2015; Michud et al. 2016).
The average tenacities of fibres prepared from acid-pretreated kraft pulp (SW-A) and eucalyptus dissolving pulp (Euca-Diss) were rather similar even though the hemicellulose content of the fibres was highly deviating, 3.9% (w/w) for Euca-DISS and 17.3% (w/w) for SW-A. This indicates that hemicelluloses do not necessarily decrease the mechanical properties of spun fibres. The average tenacities of fibres prepared from enzyme-pretreated kraft pulps (SW-E and Euca-E) were lower compared to SW-A, even though their total hemicellulose content and crystallinity indexes were rather similar. This effect could be associated with the assumed longer hemicellulose polymer length in enzyme-pretreated kraft pulps compared to acid-treatment. Additionally, the compositional analysis revealed that acid-pretreatment removed galactose substituents from glucomannan more than the enzyme-treatments and cleaved all arabinose substituents from xylan, unlike the enzyme-treatment (Table 1). Similarly, acid-pretreatment removed glucuronic acid substituents more than the enzyme-treatment detected by the decreased amount of carboxylic acids in the pulp (Table 1). Removal of arabinose substituent is shown to increase xylan affinity to cellulose in water (Köhnke et al. 2011). Chen et al. (2015b) suggested that the removal of branched hemicellulose and maintaining the less substituted hemicellulose chains would be important for the mechanical properties of the regenerated fibres. The shorter and less branched hemicelluloses might be beneficial during the spinning process and allow forming fibres with enhanced polymer alignment and increase the hydrogen bonding between cellulose and linear hemicellulose chains.
When studying the mechanical properties of the fibres further, the tenacity and elongation of the single fibres with the highest tenacities were plotted in the same graph, Fig. 4. While the average mechanical properties of regenerated fibres varied, the highest mechanical properties for every sample were similar. This indicates that it is possible to produce regenerated fibres from pretreated kraft pulps with comparable mechanical properties to fibres from dissolving pulp.
The average linear densities varied between 1.3 and 2.8 dtex. In this work the adjustment of input flow and take-up velocity had to be done during spinning, resulting in variation between the linear densities of the fibres (Table 3). A thorough study by Ma et al. (2018) suggests that the final linear density of fibres is dependent not only on the draw ratio but also on the composition of starting material and cellulose concentration in the dope. They found out that increased content of hemicellulose and lignin in the pulp resulted in thicker regenerated fibres. This may be the case also in our studies where acid- and enzyme-treated softwood pulps were spun with the same draw ratios but resulted in regenerated fibres with different linear densities (Table 3). The lower linear density of fibres from SW-A is probably due to the lower content of hemicellulose and the lower DS of hemicellulose which allowed tighter packing of the polymers.
The crystallinity index of fibres from eucalyptus dissolving pulp (Euca-Diss, 0.42) was slightly lower compared to commercial cellulosic fibres analysed by NMR: crystallinity of viscose has been reported to be 0.45–0.56 (Röder et al. 2013) and lyocell 0.44 (Zuckerstätter et al. 2013) and 0.65 (Haslinger et al. 2019). The crystallinity index of fibres from eucalyptus dissolving pulp (Euca-Diss, 0.42) was higher compared to the fibres from kraft pulps indicating that lower cellulose content in the kraft pulp-based fibres led to lower crystallinity in the fibres (0.33–0.36). Similar results on the effect of increasing hemicellulose and lignin content in the regenerated fibres on the decreasing crystallinity of regenerated fibres have been shown in a study by Ma et al. (2018), where they used unbleached birch kraft pulp for regenerated cellulose fibres.
The water uptake behaviour of the regenerated fibres was studied following the increase of fibre weight as a function of relative humidity (Fig. 5). Hydrophilicity is often a targeted property for fibres in clothing applications because it enhances the comfort of textiles on the skin. At 65% of relative humidity, fibres from the acid-treated softwood kraft pulp had the highest moisture regain (9.7% (w/w). The lowest moisture regain (7.9% (w/w) at the same humidity was measured for the fibres spun from dissolving pulp and enzyme-treated softwood kraft pulp. The higher water uptake ability of SW-A fibres compared to other hemicellulose-rich fibres might originate from differences in hemicellulose chain lengths in the pretreated pulps. The SW-A pulp is assumed to have a higher content of shorter hemicellulose chains than the other pretreated pulps. High hemicellulose content and low crystallinity were found to correlate with high water uptake ability (Table 3). This is expected since hemicellulose is shown to increase water retention (Schild and Liftinger 2014) and amorphous cellulose is known to have higher moisture uptake compared to crystalline cellulose (Garg et al. 2021).
Water uptake ability of regenerated fibres. Water uptake is shown as weight of the absorbed water in relation to the weight of dry fibres. The regenerated fibres were spun from eucalyptus dissolving pulp (Euca-Diss), acid- and enzyme-treated softwood kraft pulps (SW-A and SW-E respectively) and enzyme-treated eucalyptus kraft pulp (Euca-E)
Localisation of hemicelluloses in regenerated fibres
The location of hemicellulose in the regenerated fibres was estimated by assessing the enzymatic hydrolysis of fibres as a function of time. The dissolved monosaccharides were analysed after 1, 4 and 24 h of hydrolysis (Fig. 6) which gives an indication of the carbohydrate composition of the different layers of the regenerated fibres. Enzymatic hydrolysis is expected to proceed from the fibre surface toward the core due to the bulky size of enzyme catalysts (Rahikainen et al. 2019).
With all the regenerated fibres, the release of xylan- and glucomannan-derived sugars was faster compared to the release of cellulose-derived glucose throughout the hydrolysis time. After 24 h of hydrolysis, over 90% (w/w) of xylan and glucomannan was dissolved from the fibres spun from softwood kraft pulps while only 43% (w/w) (SW-A) and 48% (w/w) (SW-E) of cellulose was dissolved during the same time. This suggests that hemicellulose chains are enriched on the outer layers of the regenerated fibres and the fibre core is cellulose-rich. The regeneration mechanism of cellulose-hemicellulose mixtures is not yet much studied but hemicellulose is shown to have a higher solubility in IL-water mixtures than cellulose (Stepan et al. 2016a, b). Therefore, the cellulose-rich core might originate from a faster cellulose regeneration rate compared to hemicellulose.
The hydrolysability of fibres from eucalyptus kraft pulp (Fibres Euca-E) was lower compared to fibres from softwood kraft pulp. This could originate from the higher crystallinity of fibres from eucalyptus kraft pulp (Table 3). After 24 h of hydrolysis, 34% of cellulose, 86% of xylan and 64% (w/w) of glucomannan had dissolved from the fibres. The release of hemicellulose was faster compared to cellulose similarly as for the softwood samples indicating enrichment of hemicellulose, especially xylan on fibre surfaces. Fibres spun from eucalyptus dissolving pulp had the slowest release rate for hemicellulosic sugars suggesting that hemicellulose was not enriched on the fibre surface as strongly as in the hemicellulose-rich fibres. Similar results on xylan enrichment on the surface of regenerated fibres are reported earlier for commercial viscose, modal and lyocell fibres (Sjöberg et al. 2005).
Sugars liberated in enzymatic hydrolysis (peeling) of regenerated fibres after 1, 4 and 24 h. Enzymatic hydrolysis of the fibres is expected to proceed from the fibre surface to the core. Faster release of glucomannan and xylan compared to cellulose suggests enrichment of these polymers at the outer layers of the fibre structure. The regenerated fibres were spun from eucalyptus dissolving pulp (Euca-Diss), acid- and enzyme-treated softwood kraft pulps (SW-A and SW-E respectively) and enzyme-treated eucalyptus kraft pulp (Euca-E)
Regenerated fibres were imaged with confocal laser scanning microscopy to assess the morphology of fibres and location of xylan in the fibres (Fig. 7). All spun fibres were round in shape, however, considerable variation in fibre widths between fibre samples and within the samples was observed, which can also be seen from the linear densities (dtex) and their standard deviations (Table 3). In particular, regenerated fibres from enzyme-pretreated eucalyptus kraft pulp, that were spun with the lowest draw ratio, had high linear density with a large variation which correlated with the detected fibre widths.
Xylan in the regenerated fibres was localized with immunolabelling (Fig. 7) using a monoclonal antibody (CCRC-M138) that binds to an unsubstituted linear stretch of eight unsubstituted xylosyl residues (Thorne et al. 2023). Xylan was found on fibre surfaces but also from the inner layers of fibres indicated by fibre ends that appeared red after immunolabelling (circled in Fig. 7). The immunolabelling method was highly sensitive in detecting xylan since faded fluorescence signal from xylan binding antibody was detected even from the cotton linters with very low (0.4% (w/w) xylan content.
The strongest xylan signal was detected from fibres spun from acid-treated softwood kraft pulp (Fibres SW-A), although the xylan content was similar (8.2% (w/w) compared to fibres from enzyme-treated softwood kraft pulp (8.4% (w/w), Fibres SW-E) and lower compared to fibres from enzyme-treated eucalyptus kraft pulp (18.0% (w/w), Fibres Euca-E). In this work, acid-pretreatment was shown to remove substituents from the polymeric structure of xylan and immunolabelling of xylan supported this finding as acid-pretreated fibres showed enhanced antibody binding and resulted in stronger signal in the imaging. Surprisingly, the xylan rich (18.0% (w/w) fibres spun from enzyme-pretreated eucalyptus kraft pulp (Fibres Euca-E) showed weaker xylan signal than the other kraft pulp-based fibres. The reason for this is unknown and cannot be explained by the substitution degree of eucalyptus xylan which is reported to be roughly 1 in every 10th xylosyl residue (Magaton et al. 2011). Since xylan was detected from all fibres with the CCRC-M138 antibody, it suggests that processing using [mTBNH][OAc] does not cause extensive degradation or chemical modification of xylan in general. In contrast, the antibody failed in detecting xylan from commercial lyocell fibres, despite of reasonable xylan content (2.3% (w/w) suggesting that xylan in the commercial fibres is highly degraded or modified so that antibody binding does not occur (data not shown).
Confocal laser scanning microscopy images of cotton linter reference and regenerated fibres after cellulose dyeing with cellulose-binding calcofluor and immunolabelling of xylan. The regenerated fibres were spun from eucalyptus dissolving pulp (Euca-Diss), acid- and enzyme-treated softwood kraft pulps (SW-A and SW-E respectively) and enzyme-treated eucalyptus kraft pulp (Euca-E). Scale bar in the images is 200 μm. The used monoclonal antibody (CCRC-M138) binds to eight unsubstituted xylosyl residues
Conclusions
Paper-grade pulps from softwood and eucalyptus were successfully used to spin regenerated textile fibres with high hemicellulose content (17–19% (w/w)) using a process based on direct pulp dissolution in superbase ionic liquid [mTBNH][OAc]. Spinning dope with acceptable rheology for spinning was obtained after pulp pretreatment with sulfuric acid or endoglucanase enzyme. Yield loss due to pulp pretreatment and fibre spinning was estimated to be at a low level (2–3% (w/w)). The quantity of hemicelluloses was not found to impair dry tenacities or elongation since similar mechanical properties were measured from fibres with high (17% (w/w)) and low (4% (w/w)) hemicellulose content. Fibres spun after acid-pretreatment had better mechanical properties compared to fibres spun using enzyme-pretreatment. Acid-pretreatment was shown to remove hemicellulose substituents and assumed to decrease hemicellulose chain length which may have positive implications for the mechanical properties of spun fibres. Analytical peeling of the regenerated fibres suggests that hemicelluloses are enriched to the outermost layers of the regenerated fibres whereas the fibre core is cellulose-rich. Xylan was successfully imaged from the regenerated fibres using a xylan backbone-binding antibody. This suggests that the used ionic liquid does not introduce major chemical modifications to xylan nor degrade it to a large extent.
Harmful side reactions that take place with hemicellulose and solvent chemicals in commercial fibre spinning processes have prevented the use of hemicellulose-rich raw materials in the production of regenerated fibre products. This work demonstrates that a broader raw material base for regenerated textile fibres may be reached with novel types of solvents (superbase ionic liquids) that enable the regeneration of both cellulose and hemicellulose in high yield and do not react extensively with hemicellulose.
References
Alén R (2000) Structure and chemical composition of wood. In: Stenius P (ed) Forest products Chemistry. Fapet Oy, Jyväskylä, pp 12–55
Alén R (2011a) Principles of biorefining. In: Alén R (ed) Biorefining of forest resources. Paper Engineers’ Association, Helsinki, pp 55–114
Alén R (2011b) Structure and chemical composition of biomass feedstocks. In: Alén R (ed) Biorefining of Forest resources, 1st edn. Paper Engineers’ Association, Helsinki, Finland, pp 17–54
Azimi B, Maleki H, Gigante V et al (2022) Cellulose-based fiber spinning processes using ionic liquids. Cellulose 29:3079–3129
Balkissoon S, Andrew J, Sithole B (2022) Dissolving wood pulp production: a review. Biomass Conversion and Biorefinery 1:10. https://doi.org/10.1007/s13399-022-02442-z
Berggren R, Berthold F, Sjöholm E, Lindström M (2001) Fiber strength in relation to molecular mass distribution of hardwood kraft pulp degradation by ozone and acid hydrolysis. Nord Pulp Pap Res J 16:333–338. https://doi.org/10.3183/npprj-2001-16-04-p333-338
Berthold F, Gustafsson K, Berggren R et al (2004) Dissolution of softwood kraft pulps by direct derivatization in lithium chloride/N,N-dimethylacetamide. J Appl Polym Sci 94:424–431. https://doi.org/10.1002/app.20697
Chen JH, Guan Y, Wang K et al (2015a) Regulating effect of hemicelluloses on the preparation and properties of composite lyocell fibers. Cellulose 22:1505–1516. https://doi.org/10.1007/s10570-015-0608-0
Chen JH, Wang K, Xu F, Sun RC (2015b) Effect of hemicellulose removal on the structural and mechanical properties of regenerated fibers from bamboo. Cellulose 22:63–72. https://doi.org/10.1007/s10570-014-0488-8
El Seoud OA, Kostag M, Jedvert K, Malek NI (2020) Cellulose regeneration and chemical recycling: closing the cellulose gap using environmentally benign solvents. Macromol Mater Eng 305:1900832
Elsayed S, Hellsten S, Guizani C et al (2020) Recycling of superbase-based ionic liquid solvents for the production of textile-grade regenerated cellulose fibers in the lyocell process. ACS Sustain Chem Eng 8:14217–14227. https://doi.org/10.1021/acssuschemeng.0c05330
Fulcher RG, Irving DW, De Francisco A (1989) Fluorescence microscopy: applications in food analysis. In: Munck L (ed) Fluorescence analysis in foods. Longman Scientific and Technical, Harlow, pp 59–109
Garg M, Apostolopoulou-Kalkavoura V, Linares M et al (2021) Moisture uptake in nanocellulose: the effects of relative humidity, temperature and degree of crystallinity. Cellulose 28:9007–9021. https://doi.org/10.1007/s10570-021-04099-9
Gericke M, Fardim P, Heinze T (2012) Ionic liquids - Promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17:7458–7502
Haslinger S, Hietala S, Hummel M et al (2019) Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydr Polym 207:11–16. https://doi.org/10.1016/j.carbpol.2018.11.052
Hauru LKJ, Hummel M, King AWT et al (2012) Role of solvent parameters in the regeneration of cellulose from ionic liquid solutions. Biomacromol 13:2896–2905. https://doi.org/10.1021/bm300912y
Köhnke T, Östlund Ã, Brelid H (2011) Adsorption of arabinoxylan on cellulosic surfaces: influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromol 12:2633–2641. https://doi.org/10.1021/bm200437m
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma Y, Hummel M, Määttänen M et al (2016) Upcycling of waste paper and cardboard to textiles. Green Chem 18:858–866. https://doi.org/10.1039/c5gc01679g
Ma Y, Stubb J, Kontro I et al (2018) Filament spinning of unbleached birch kraft pulps: effect of pulping intensity on the processability and the fiber properties. Carbohydr Polym 179:145–151. https://doi.org/10.1016/j.carbpol.2017.09.079
Magaton AS, Colodette JL, Piló-Veloso D, Gomide JL (2011) Behavior of eucalyptus wood xylans across kraft cooking. J Wood Chem Technol 31:58–72. https://doi.org/10.1080/02773813.2010.484123
Mäki-Arvela P, Anugwom I, Virtanen P et al (2010) Dissolution of lignocellulosic materials and its constituents using ionic liquids — a review. Ind Crop Prod 32:175–201. https://doi.org/10.1016/j.indcrop.2010.04.005
Martins MAR, Sosa FHB, Kilpeläinen I, Coutinho JAP (2022) Physico-chemical characterization of aqueous solutions of superbase ionic liquids with cellulose dissolution capability. Fluid Phase Equilib 556:113414. https://doi.org/10.1016/j.fluid.2022.113414
Massiot D, Fayon F, Capron M et al (2002) Modelling one- and two-dimensional solid-state NMR spectra. Magn Reson Chem 40:70–76
Michud A, Hummel M, Sixta H (2016) Influence of process parameters on the structure formation of man-made cellulosic fibers from ionic liquid solution. J Appl Polym Sci 133:app.43718. https://doi.org/10.1002/app.43718
Moriam K, Sawada D, Nieminen K et al (2021) Towards regenerated cellulose fibers with high toughness. Cellulose. https://doi.org/10.1007/s10570-021-04134-9
Potthast A, Radosta S, Saake B, et al (2015) Comparison testing of methods for gel permeation chromatography of cellulose: coming closer to a standard protocol. Cellulose 22:1591–1613. https://doi.org/10.1007/s10570-015-0586-2
Rahikainen J, Ceccherini S, Molinier M et al (2019) Effect of cellulase family and structure on modification of wood fibres at high consistency. Cellulose 26:5085–5103. https://doi.org/10.1007/s10570-019-02424-x
Rahikainen J, Mattila O, Maloney T et al (2020) High consistency mechano-enzymatic pretreatment for kraft fibres: effect of treatment consistency on fibre properties. Cellulose 27:5311–5322. https://doi.org/10.1007/s10570-020-03123-8
Röder T, Moosbauer J, Wöss K et al (2013) Man-made cellulose fibres-a comparison based on morphology and mechanical properties. Lenzing Ber 91:7–12
Rosenau T, Potthast A, Adorjan I et al (2002) Cellulose solutions in N-methylmorpholine-N-oxide (NMMO) - degradation processes and stabilizers. Cellulose 9:283–291. https://doi.org/10.1023/A:1021127423041
Rosenau T, Potthast A, Hofinger A et al (2002) Instabilities in the system NMMO/water/cellulose (lyocell process) caused by polonowski type reactions. Holzforschung 56:199–208. https://doi.org/10.1515/HF.2002.033
Schild G, Liftinger E (2014) Xylan enriched viscose fibers. Cellulose 21:3031–3039. https://doi.org/10.1007/s10570-014-0302-7
Shi H, Wan Y, Li O, et al (2020) Two-step hydrolysis method for monosaccharide composition analysis of natural polysaccharides rich in uronic acids. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2019.105524
Singh SC, Murthy ZVP (2017) Study of cellulosic fibres morphological features and their modifications using hemicelluloses. Cellulose 24:3119–3130. https://doi.org/10.1007/s10570-017-1353-3
Sixta H (2006) Pulp properties and applications. In: Sixta H (ed) Handbook of pulp, vol 2. Wiley-VCH, New York, pp 1009–1067
Sixta H, Michud A, Hauru L et al (2015) Ioncell-F: a high-strength regenerated cellulose fibre. Nord Pulp Pap Res J 30:43–57
Sjöberg J, Potthast A, Rosenau T et al (2005) Cross-sectional analysis of the polysaccharide composition in cellulosic fiber materials by enzymatic peeling/high-performance capillary zone electrophoresis. Biomacromol 6:3146–3151. https://doi.org/10.1021/bm050471j
Sluiter A, Hames B, Ruiz R et al (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced (LAP)
Spönla E, Rahikainen J, Grönqvist S, Potthast A (2023) High consistency enzymatic pretreatment of eucalyptus and softwood kraft fibres for regenerated fibre products. Cellulose. https://doi.org/10.1007/s10570-023-05144-5
Stepan AM, Michud A, Hellstén S et al (2016) IONCELL-P&F: pulp fractionation and Fiber spinning with ionic liquids. Ind Eng Chem Res 55:8225–8233. https://doi.org/10.1021/acs.iecr.6b00071
Stepan AM, Monshizadeh A, Hummel M et al (2016) Cellulose fractionation with IONCELL-P. Carbohydr Polym 150:99–106. https://doi.org/10.1016/j.carbpol.2016.04.099
Sturm M, Helminen JKJ, Honkasalo A et al (2023a) Investigations for the use and recyclability of the ionic liquid [MTBDH][AcO] as a solvent in air-gap-wet spinning process. J Appl Polym Sci. https://doi.org/10.1002/app.53901
Sturm M, Kosan B, Kilpeläinen I (2023b) Towards new solvents for Lyocell—a comparison of [MTBDH][AcO] and its successor [MTBNH][AcO], Poster at Cellulose Fibres Conference, Cologne, Germany
Su P, Granholm K, Harju L, Ivaska A (2011) Binding affinities of different metal ions to unbleached hardwood kraft pulp. Holzforschung 65:619–622. https://doi.org/10.1515/HF.2011.080
Tenkanen M, Siika-Aho M (2000) An α-glucuronidase of schizophyllum commune acting on polymeric xylan. J Biotechnol 78:149–161. https://doi.org/10.1016/S0168-1656(99)00240-0
Thorne K, Urbanowicz BR, Hahn MG (2023) Plant cell wall glycan-directed monoclonal antibodies. In: Geitmann A (ed) The plant cell wall – research milestones and conceptual insights. CRC Press, Taylor & Francis Group, Boca raton
Willberg-Keyriläinen P, Ropponen J, Lahtinen M, Pere J (2019) Improved reactivity and derivatization of cellulose after pre-hydrolysis with commercial enzymes. BioResources 14:561–574. https://doi.org/10.15376/biores.14.1.561-574
Zhang H, Zhang H, Tong M et al (2008) Comparison of the structures and properties of lyocell fibers from high hemicellulose pulp and high α-cellulose pulp. J Appl Polym Sci 107:636–641
Zuckerstätter G, Terinte N, Sixta H, Schuster KC (2013) Novel insight into cellulose supramolecular structure through 13 C CP-MAS NMR spectroscopy and paramagnetic relaxation enhancement. Carbohydr Polym 93:122–128. https://doi.org/10.1016/j.carbpol.2012.05.019
Acknowledgments
This research has received funding from the Bio-based Industries Joint Undertaking (JU) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No 837527. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and the Bio-based Industries Consortium. Prof. Ilkka Kilpeläinen, University of Helsinki, is acknowledged for providing ionic liquid for the study. Pulp-producing companies, Metsä Fibre and Altri group are acknowledged for providing pulp raw materials for the study. Research Scientist Lotta Sorsamäki is acknowledged for mass balance calculations to estimate yields and Principal Scientist Marjo Määttänen is acknowledged for planning acid-pretreatments for pulp. Technical assistance from Liisa Änäkäinen, Nina Vihersola, Hanna Karola and Mariitta Svanberg is gratefully acknowledged. Dr, Sonja Schiehser is acknowledged for the help conducting molar mass analysis.
Funding
Open Access funding provided by Technical Research Centre of Finland. Funding for open access publishing was provided by the Technical Research Centre of Finland (VTT). This research has received funding from the Bio-based Industries Joint Undertaking (JU) under the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 837527. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and the Bio-based Industries Consortium.
Author information
Authors and Affiliations
Contributions
ES, JR, TK and SH wrote the main manuscript text. UH contributed to the antibody labelling and imaging of the regenerated fibres and the text concerning them. IS and AP provided NMR and SEC results, Fig. 2 and contributed to the text. Other figures were drawn by ES (1, 3, 6, 7), SH (4) and TK (5). SG and AH participated in the investigation and validation. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have given their consent for publishing.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spönla, E., Hannula, S., Kamppuri, T. et al. Hemicellulose-rich paper-grade pulp as raw material for regenerated fibres in an ionic liquid-based process. Cellulose 30, 11407–11423 (2023). https://doi.org/10.1007/s10570-023-05589-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05589-8