Abstract
This work deals with enhancing the rice straw as precursor of cellulose-based nanoparticles in further application in production of nanogels for prolonging the bioactive compound (Carvedilol drug) release time (sustained release), till the release of the drug is continuous and useful, with minimal side effects. The benefit of the investigated nanogels was proved from comparing their sustaining release with patent and literature data. The nanogels were synthesized from chelating of cellulose-nanoparticles (ONC) in hybrid with palm extract (PE) by chitosan. The effective role of pulping reagent to prepare the pulp precursor of ONC was also assessed. The ONCs were prepared from different RS-pulp precursors, using soda, soda-anthraquinone-borohydride and AcOH-organosolv pulping reagents. FT-IR, SEM, non-isothermal TGA and swelling behavior of nanogels, in addition to their in vitro release of drug and kinetics were studied. The data showed that incorporating the PE to ONCs, especially from alkaline pulp-produced nanogels with highestsustaining behavior, where at 5 h the release % was decreased from ~ 50 to 33% and provided 50% CAR release at 24 h. This time was greater than reported by literature on using other drug carriers (10–18 h), which will stimulate the reduction of the number of doses. Almost all PE-containing gels followed the zero-order kinetic release.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on environmental legislation to reduce the pollution which results from burning of non-wood lignocellulosic wastes as traditional route to dispose it, our research work and other researchers investigated diverse applications of these wastes, e.g., activated carbon, carbon nanotubes, cellulose derivatives for cancer treatment, functional paper, and wood products, as well as hydrogels for agricultural purposes, and optical materials, (Lavanya et al. 2011; Thakur et al. 2013; Fathy et al. 2017; Lotfy et al. 2018; 2020; Ho et al. 2021; Basta 1998; Basta et al. 2015, 2017, 2021a, b).
The drug delivery system has been investigated since about 1952, and it was known as a formula for introducing the required drug into the body. These systems are based on Synthetic or biopolymers. Examples of synthetic polymers are Conjugation of poly (styrene-co-maleic acid) derivatives, poly(oxyethylene)-poly(oxypropylene) block copolymers، poly (ethylene oxide-aspartate) block copolymer-adriamycin conjugates, Low- and high molecular-weight polyethylenimine, poly(ε-caprolactone) (Maeda et al. 1985; Kabanov et al. 1992; Kunath et al. 2003; Shuai et al. 2004). With regard to biopolymers, it depends on protein origins (e.g., Silk, collagen, gelatin and albumin (Maham et al. 2009; Gopi et al., 2016; Xie et al. 2016; Perteghella et al. 2017; Salerno et al. 2018; Fu et al. 2018; Farokhi et al. 2018), or carbohydrates (e.g., chitosan, alginate, crystalline cellulose, and starch [(MacLaughlin et al. 1998; Babu et al. 2007; Zhao et al. 2012; Dafe et al. 2017; Cong et al. 2018), and have attracted much attention than synthetic due to their biodegradation and homogeneity behavior, Moreover, poly(co-lactide-glycolic acid)nanoparticles and poly(ethylene-co-vinyl acetate) nanoparticles coated with chitosan] are used as nanomaterials to release the drug (Varshosaz et al. 2013; Pal et al. 2018).
Carvedilol is a drug used to treat the heart, such as high blood pressure and its weakness in dysfunction. Unfortunately, its disadvantages are focused on lack of solubility and absorption. Literature reported the release formulations in tablet form of this drug with cornstarch, Methocel K15M, Povidone K30, magnesium Stearate (Hao et al. 2010). Another formulation also in tablet form (Kumaret al. 2017) included Carvedilol, Methocel K4M CR, Methocel K15M CR, starch, Avicel PH101, talc and magnesium stearate. This later tablet provided time of release 25% and 50% at 3.31 and 9.88. Regarding the application of cellulose derivatives, the investigators found that the addition of cellulose ether (Hydroxypropyl methylcellulose) in the components of a 180 mg tablet consisting of (carvedilol, cellulose ether, lactose, magnesium stearate, crystalline cellulose and polyvinylpyrrolidone) from 27 to 60 mg increasing the release time of 50% carvedilol from 4.5 to 18.72 h (Ashkari et al. 2013).
This work was done for valorization of rice straw to investigate a simple control release system for Carvedilol which is characterized by sustaining release. In this respect different Rice straw pulps were prepared, using three pulping reagents (Soda, Soda-Anthraquinone-Sodium Borohydride, AcOH-based organosolv), and further oxidation by ammonium persulfate to produce nanocelluloses (ONCs). Hybrid ONC with palm extractives were chelated by chitosan. The swelling behavior, FT-IR spectra, SEM, and in vitro release of drug from investigated systems, and kinetics were studied. The effective role of palm extractives on sustaining release was assessed by comparing it with PE free systems.
Materials and methods
Preparation of nanocelluloses (ONCs)
The preparation of oxidized nanocelluloses (ONCs) was performed in two stages; first by produced rice straw pulps via using three pulping reagents (NaOH without or with anthraquinone (AQ) and sodium borohydride (BH) additives, as well as AcOH-organosolv reagents. RS was subjected to pretreatments by hot water and screening before pulping at 120 °C for 1 h and liquor ratio 6/1. The holocellulose content of bleached pulps were 77.6%, 76.4% and 61.6%, respectively. Second, the washed pulps were oxidized with ammonium persulfate according to the method reported in Reference (Filipova et al. 2018) with some modifications. The oxidized nanocelluloses with different carboxyl contents were prepared, five grams (dry weight) were soaked in distilled water (200 mL), and then the suspension was heated to 70 °C in a water bath. Afterwards, APS (purity, 98%; 47 g), was added to the suspension. The mixture was then heated at 70 °C for 6 h with continuous stirring. The reaction was stopped by cooling the mixture in an ice bath, and then oxidized cellulose was filtered using a centrifuge and washed until it reached the pH of distilled water. The carboxyl content of the prepared oxidized CNPs was determined by conductometric titrations (Jianget al. 2013). The contents of carboxylic groups were grown from 0.145 to 0.332 µmol/g α-cellulose). The highest carboxyl content was corresponding to AcOH-organosolv pulp (332 µmol/g), followed by NaOH-AQ-BH pulp (¬ 260 µmol/g). The nanocellulose from Na2O Pulp is in the last sequence (127 µmol/g). The pulping reagents were purchased from Sigma-Aldrich, while the ammonium persulfate with purity 98% (APS) was provided from Merck. These synthesized oxidized nanocellulose from NaOH, NaOH-AQ-BH, and AcOH-organosolv pulping reagents were coded ONC (1), ONC (2) and ONC (3), respectively.
The nanostructure and degree of crystallinity of these prepared ONCs were tested using high resolution-transmission electron microscopy (HR-TEM) at 200 kV, using JEOL (JEM-1230 FX, Japan), and X-ray diffractometer using a Cu-Kα radiation (EMPYREAN, Japan). For HR-TEM, few drops of diluted aqueous sonicated ONCs suspensions were deposited on copper-coated carbon grids with 200 mesh size, allowed to dry then the sample stained with 1% phosphotungstic acid.
For XRD analysis, the relative intensities of ONCs films were recorded at a scanning rate of 50° min−1, step size 0.026° and wavelength 1.54 Å in reflection mode. The diffraction patterns were recorded with scanning ranging from 4° to 80° on a 2θ scale. The fitting range at 10–60° 2θ was used when the cellulose XRD covers all the notable crystalline peaks in two step sizes 0.026 and 0.1 to reduce the noise in the peaks. Subtracting the background before peak deconvolution led to stable baseline fitting and reduces fitting error. The fitting curves are calculated using the Origin software by applying Lorentz equation. The percentage of crystalinity can be determined using peak height peak and deconvolution methods.
Peak height method | \({\text{Crystallinity}}\,\left( {\text{\% }} \right) = { }\frac{{I_{{200{ }}} - I_{{am{ }}} }}{{I_{{200{ }}} }} \times 100\) | Thorat and Dastager (2018) |
Iam the minimum intensity between the 110 and 200 peaks (~ 18.5°) | ||
I200 the maximum intensity at 200 peak | ||
Peak deconvolution methods | \({\text{Crystallinity}}\,\left( {\text{\% }} \right) = \frac{{{\text{Area}}\,{\text{under}}\,{\text{crystalline}}\,{\text{peaks }}}}{{{\text{Total}}\,{\text{area}}\,{\text{under}}\,{\text{all}}\,{\text{peaks}}}} \times 100\) | Doumeng et al. (2021) |
The FT-IR spectra were recorded, using JASCO4600-FT/IR, United States. The samples were mixed with KBr and pressed as discs. The absorbance spectra were recorded in the wave-number range 4000–400 cm−1.
Preparation of extractive palm compound (PE)
The palm leaves were collected, washed several times, and left to dry. The dried leaves were immersed or soaked in methanol-benzene with ratio 1:1 for at least 24 h then filtered the extracted solution. The filtrate was subjected to rotary evaporation for solvents removal and complete drying occurred in the oven at 70 °C for further uses.
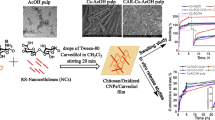
The following flow chart (Fig. 1) summarized the route for preparation and evaluation of nanogels for controlling the release of Carvedilol (CAR) which was purchased from Sigma-Aldrich.
Preparation of nanogels and drug loading
The nanogels were synthesized by the following procedure: Chitosan solutions (50 mg/mL, 10 ml) are prepared by dissolving chitosan in 1% acetic acid. Few drops of Tween-80 (2% v/v) were added as a surfactant. Carvedilol (CAR) (100 mg) is dissolved in CH2Cl2 and then this oil phase is mixed with aqueous phase (chitosan solution containing Tween) by stirring for 20 min. CH2Cl2 is chosen because of its ability to diffuse into the aqueous phase at a rapid rate facilitating particle formation upon evaporation. ONC suspension (166 mg-dry weight) was dropped into the last solution under magnetic stirring and left 24 h for complete crosslinking, followed by drying in the oven at 60 °C. The ratio of chitosan to ONC was 3:1. To assess the role of Palm extractives (PE) in sustaining the release of drug, the same procedures were repeated with substitution 50% or 100% of ONC with this extractive (hybrid nanogels). Parallel experiments were carried out in preparing nanogels free from CAR. The nanogels prepared from ONCs, using different RS-pulp precursors were coded Cs-ONC (1), Cs-ONC (2) and Cs-ONC (3); while those prepared from hybrid ONCs with PE were coded Cs-ONC (1)-PE, Cs-ONC(2-PE) and Cs-ONC (3)-PE. CAR drug-loaded gels were coded CAR-Cs-ONC and CAR-Cs-ONC-PE.
Characterization of unloaded and loaded nanocomposite hydrogels
-
FTIR spectral analysis: The prepared Cs-ONC, Cs-ONC-50 PE, Cs-PE nanogels in absence and presence of the drug were subjected to FTIR analysis using Varian 640-IR, USA spectrometer with KBr disc method. The spectra were collected in range 400–4000 cm−1 to ascribe the function groups of the prepared samples and their compatibility with the drug. The mean strength of hydrogen bond and crystallinity index were calculated according to references (Nelson and O’Connor 1964; Levdik et al. 1967; Abdel-Hadi et al. 1994).
-
Scanning electron microscopy (SEM) The surface morphology of unloaded and drug loaded nanocomposite hydrogels were examined, after coating the samples with gold, using a scanning electron microscope, quanta FEG250 system, running the electron beam at 20 kV accelerating voltage.
-
Thermogravimetric analysis (TGA) was carried out for the pure and the loaded nanocomposite hydrogels using Setaram LABSYS EVO STA, France. All measurements were achieved over a temperature range of 30–1000 °C using a heating scan rate of 10 °C/min in an inert atmosphere of nitrogen gas (30 ml/min). All measurements were achieved over a temperature range of 30–1000 °C using a heating scan rate of 10 °C/min in an inert atmosphere of nitrogen gas (30 mL/min). The Kinetic parameters of thermal degradation were calculated according to References (Coat and Redfern 1964; Basta et al. 2008, 2016 and El-Saied et al. 1994).
Evaluation of nano-based gel hydrogels as drug delivery systems
Swelling measurements
The swelling behavior of the dried nanocomposite hydrogels was performed by immersing the gels in methanol-phosphate buffer with ratio (3: 1) as reported previously (Hannaet al. 2020; Basta and Lotfy 2021). Briefly, 0.1 g of dry powder gel samples were placed into a weighted nylon tea bag and immersed in 50 mL buffer solutions, at room temperature. After regular time intervals, the hydrogels were removed from the aqueous solution and weighed after removing the excess surface water. The swelling capacity (% Q) was calculated using the following Eq. (1)
where Ws and Wd are the weight of swollen and dry gels.
Three replicates were conducted, and the average values were presented.
In vitro release studies
The release behavior of the loaded nanocomposite hydrogels was tested in methanol-buffer solutions. The loaded hydrogel samples (0.1 g) were placed in 50 mL buffers at 37 °C and 50 rpm in a shaking incubator. 0.5 mL solution was periodically removed at different interval times from 1.0 to 48.0 h and the amount of CAR was analyzed at 285 nm by spectrophotometry (Ajun et al. 2009). In order to maintain the original buffer volumes of 50 ml, 5 ml fresh buffer solution was periodically added. All measurements were performed in triplicate, and the CAR drug released was evaluated by using equation:
Kinetic study
Four different models namely, Ritger and Peppas, zero-order, first order and Higuchi were used to study the kinetic of release the CAR from the synthesized chitosan-oxidized CNPs (Kajalet al. 2012; Xu et al. 2015; Gouda et al. 2017).
Results and discussion
Characterization of oxidized nanocelluloses (ONCs)
The Transmission electron microscope (TEM) images of the prepared ONCs, using different RS pulps precursors are presented in Fig. 2. The suspension of samples was diluted in water and stained by the phosphotungstic acid as negative staining. As shown in Fig. 2, the morphological features of ONCs were observed obviously as spindle or needle like shape. The TEM images depict agglomeration of ONC bundles, points with dispersed crystallites, and individual crystals. The width of selected oxidized CNPs was defined as the largest dimension measured across the CNPs, and its values ranged from 7.3 ± 1.4 to 15.7 ± 2.4 nm. Meanwhile, the length was also measured along the CNPs long axis and ranged from 137.3 ± 41.9 to 250.2 ± 20.2 nm. Table 1 recorded their length and width beside the aspect (length-to-width) ratio which calculation ranged from 15.88 to 19.28. These aspect ratios were lower than nanocelluloses from other cellulosic sources, such as wood (35–38) (Roman and Gray 2005), ramie (25–42) (Habibiet al. 2008), sisal (43) (Siqueira et al. 2010), bacteria (60–80) (Olssonet al. 2010) and tunicate (251–493) (Iwamotoet al. 2009).
The XRD patterns in Fig. 3 show that, all the diffractograms of CNP samples possessed reflections at 16.8° and 22.7° assigned to (110) and (200) crystallographic planes (French 2014; Moraiset al. 2013).. The crystallinity ratio (Cr. ratio), d-spacing and the apparent crystallite size for the reflection planes of the ONC samples were calculated and presented in Table 2. According to the peak height method, the crystallinity of ONC samples ranged from 79.1 to 92.6%. In an agreement with (Park et al. 2010), the crystallinity values from the peak height method are significantly higher and depend on time-saving empirical measure of relative crystallinity. So, XRD deconvolution method is used to determine the crystallinity. As shown, the XRD-crystallinity ratio values of ONC (2) and ONC (3) of alkali pulps (NaOH-AQ-BH and organosolv reagents) noticeably higher (64.44 and 63.62%) than that resulted from NaOH reagent; ONC (1) (53.49%). This indicates that the soda pulping condition causes partial destruction of crystalline areas of cellulose macromolecules. Additionally, when BH additives are used to NaOH pulping, the crystallinity ratio values are improved in comparison to NaOH pulping. Compared with other literature, the crystallinity index of RS-extracted cellulose treated with APS ranges from 30 to 50% (Oun and Rhim 2018). The nanocrystalline cellulose prepared from other biomasses such as the balsa and kapok fibers using APS showed crystallinity index 43–78% and 51–81%, respectively (Marwanto et al. 2021). Moreover, the CNCs from an empty fruit bunch of palm oil waste reached a crystallinity index 64% using a one-step extraction method with minimum APS concentration of 1.5 M (Madani et al. 2022). So, the crystallinity of our investigated case using peak height method (79.1–92.6%) or deconvolution method (53.49–64.44%) of pulped RS showed higher values as compared with many previous publications.
The analysis of the XRD pattern allows us to determine the average size of the crystallites using Scherrer ́s formula. The average size of the crystallite for the reflection plane (200) is ranged from 2.82 to 3.39 nm. This evidenced that the APS oxidation was not the same affected on the pulp precursors but produced nanocelluloses with different crystallite size. The non-similarity in crystallite size of plane (200) inferred that the hydroxyl groups at C2 and C3 might be detectably oxidized by APS (Jiang et al. 2017). Moreover, these values are lower than those reported for crystalline cellulose nanoparticles obtained from sugarcane bagasse (Teixeiraet al. 2011), Cereus Forbesii (Orrabalis et al. 2019), Agabe angustifolia fibers (Rosli et al. 2013), coconut husk (Rosaet al. 2010), and Sisal fibers (Juanet al. 2008). Additionally, Table 2 showed the crystallites of the ONCs have a cross section with widths of varied from 2.82 to 4.65 nm, corresponding to 110 planes (Jiang et al. 2017).
Regarding the FTIR spectra test, which presented in Fig. 4, the main changes that occurred on oxidation of cellulose from RS-pulps than the control cellulose are noticed in red shifting of O–H stretching vibration from 3421 cm−1 to lower value ranged from 3401 to 3378 cm−1, moreover with appearing of a peak at 1730 cm−1 for APS-CNPs which is related to the C=O stretching vibration. This shifting with presence of C=O confirmed that the hydroxyl groups of the cellulose pulps have been oxidized to carboxyl groups during APS treatment. It was reported that the presence of carbonyl groups on the surface of CNPs would make them more reactive and improve their properties needed for their use in nanocomposites (Cheng et al. 2014).
Characterization of unloaded and loaded nanocomposites
FTIR spectral analysis
Regarding drug free nanocomposites, formulations of Cs chelated with ONCs didn’t show variations in their spectra when compared with a pure Cs spectrum (Fig. 5a). This indicated absence of covalent bond between Cs and ONCs, and possibly formation of hydrogen bonding with electrostatic interaction. Because of the stretching vibration of O–H and N–H the band of the Cs is shifted to lower wavenumbers from 3454 to 3416 cm−1 by chelating of ONC suggesting weak hydrogen bonds interaction happened between these two components as well as strong interaction between carboxyl groups (COO−) of ONCs with NH3+ of chitosan. Moreover, the C=O band is shifted to lower wavenumber (1538–1626 cm−1) as compared with Cs and ONCs (1639 cm−1). For the case of preparation of nanogels from chelating hybrid ONC with Palm extract (PE) (Fig. 5b), showed no additional band as compared with Cs-ONCs nanogels due to the PE has the same characteristic bands; which are broad peak ~ 3400 cm−1 (the stretching vibration of OH groups of phenolic and polyphenolic constituents), bands ~ 2925 cm−1 (stretching vibration of C–H of aromatic skeletons like flavonoids or aromatic acids), band at 1650 cm−1 (C=C stretching of aromatics and other carbonyl C=O stretching of flavonoids or polyphenols). Other characteristic peaks related to stretching of C–C, bending vibration of C–H in aromatic rings and C–O group of polyols appeared at 1450, 1500 and 1250 cm−1 (Khalil et al. 2021). The peak at 1735 cm−1 represented the C=O of the ester group found in tween 80. The red shift of OH/NH band of chitosan in the range 4000–3000 cm−1 indicates that the chemical interaction suggested for Cs-ONC-PE nanogels with physical hydrogen bonding interaction. The peaks in the fingerprint area of Cs-CNPs-EPC were stronger than Cs-CNPs nanocomposites due to the interfering bands of all the components making the peaks stronger.
The ratio of absorbance of –OH or/and –NH stretching to that of C-H stretching is called the mean strength of hydrogen bonds (MHBS), given in Table 3. It can be noticed that the MHBS values of the synthesized gel of nanocelluloses were higher than those of their components especially for Cs-PE and Cs-ONC (1)-PE. This suggests that the functional groups including the components of the gels are predominately shared in hydrogen bonds formation between the hydroxyl of phenolic compounds or ONCs and NH2 of Cs rather than ionic bonds. On comparison the crystallinity index (Cr. I), which was determined from FT-IR spectra for nanogels and their components (Table 3), it revealed that hydrogels exhibited mostly lower values than those of chitosan and usually higher than the oxidized celluloses.
After drug loading, the FTIR spectra of the drug loaded nanogels showed no additional band, irregular changes of the position and intensity of OH/NH group, some little changes of MHBS and Cr.I. as compared to drug free nanocomposites. The bands found at the fingerprint area of the drug loaded nanocomposites become sharper with high intensity especially for bands at ~ 1100 and 600 cm−1 The sharp bands at the fingerprint region are from the characteristics of CAR drug, this indicates the loading of CAR on the hydrogels. After drug loading, the H-bond formation between molecular chains of loaded nanocomposites was reduced in comparison with unloaded one. With regards to the crystallinity index (Cr. I) (Table 3), it revealed that nanogels exhibited mostly lower values than those of the unloaded nanocomposites.
Surface morphology (SEM)
The morphological structure of the investigated nanogels before and after CAR drug loading was determined by SEM. Figure 6a demonstrated the SEM micrographs of drug Free-Cs with oxidized ONCs individually and in hybrid with PE nanogels and drug loaded samples. For drug-free gels, it is clear the rough homogeneous surface free of needle crystals of Cs-PE gel, which indicated a good compatibility between the Cs polysaccharide and the PE phenolic constituents due to the H-bonding interaction. After drug loading, the surface roughness increased due to covering the gel with white particles of CAR drug. The CAR drug appeared in randomly distributed white particles with different grain size discrepancy in the nanocomposite matrix (Sip et al. 2021).
a SEM images of nanogels from unloaded and loaded drug of chitosan (CS) with hybrid oxidized nanocellulose (ONC) with palm extract (PE) carriers from A Cs-PE, B Cs-PE-CAR C Cs-ONC (1), D Cs-ONC (1)-PE, E Cs-ONC (1)-PE-CAR. b SEM images of nanogels from unloaded and loaded drug of chitosan (CS) with hybrid oxidized nanocellulose (ONC) with palm extract (PE) carriers from F Cs-ONC (2), G Cs-ONC (2)-PE, H Cs-ONC (2)-PE-CAR, I Cs-ONC (3) J Cs-ONC(3)-PE and K Cs-ONC(3)-PE-CAR
For drug-free nanogels of Cs with oxidized NC showed rough surface of needle-shaped oxidized CNPs dispersed and the random oriented in the Cs matrix with no phase separation. With hybrid oxidized NC with PE to form Cs-ONC-PE gels (Fig. 6a, b), the surface becomes smoother without any pores or cracks and with good structural integrity. This indicates a good interfacial adhesion between all components which is attributed to strong interactions between nanocellulose, palm extractive and chitosan, that is caused by the ionic/hydrogen bonding between the and functional groups (OH, COO, NH3+). The micrographs of all nanocomposites gels after drug loading illustrate CAR drug emerged like white dots on the surface of gels. These bright dots can be seen in random distributions with different grain sizes.
Thermogravimetric analysis (TGA)
The role of palm extract (PE) on thermal stability of the synthesized nanogels (Cs-PE and Cs-ONCs-PE) was studied in comparison with those free from PE (Cs-ONCs); Fig. 7a,b and Table 4a,b. On comparing both types of nanogels with ONCs, it is noticed that they have two degradation stages, the first related to evolution of adsorbed water, while the second is related to volatilization stage (main degradation) as a result of degradation of the main components of the gels with evolution of gasses. For ONCs this second stage was noticed in DTG as one peak; while for nanogels as 2 peaks, with changing the DTG peak temperature and activation energy (Ea) according to type of ONC precursor (RS-pulp), as well as PE included the nanogels. one related to degradation of bond formation between These peaks are related to degradation of chelating bonds formed between chitosan and ONCs and another due to the degradation of matrix of polymer gel.
As can be seen that, the main degradation stage of the nanogels from chelating chitosan with palm extract (Cs-PE) has lower DTG peak temperatures (266.7 and 408.5 °C) and activation energy (ΣEa = 235.94) than nanogels of Cs-ONCs (DTG peaks 296–302 °C ranged 413–418 °C; while ΣEa ranged from 327.33 to 380 kJ/mole). This indicates that the degree of interaction between ONCs with chitosan is higher than hybrid ONC-PE. Due to this behavior the DTG peaks and Ea of nanogels were decreased on chelating Cs with hybrid PE-ONCs (Cs-ONCs-PE nanogels); Table 4a.
On loading the Carvedilol drug, the TGA measurements of most nanogels were decreased than unloaded one. This indicates that other types of bonds are formed between functional groups of drugs with gel components, rather than ionic bonds, which play a role to restrict it from releasing (Table 4b).
Evaluation of nanocomposites as drug delivery systems
Swelling measurements
Figure 8 illustrates the effect of the ONCs substitution partially or completely by palm on swelling behavior of produced gels. The gels from chelating extractive with Cs remarkably increased the hydrogel swelling ratio. The swelling ratios of Cs-ONCs ranged from 142.5 to 165.3% after 24 h, while the swelling ratio of Cs-PE nanocomposite films was 232.8% that led to deterioration of the buffer resistivity. This swelling ratio might be for the hydrogen bonding between the hydroxyl groups of Cs molecules and PE phenolic compounds in the composite gels. So, the gel containing plenty of functional groups, such as amide and hydroxyl groups on the surface, could dramatically increase the density of the hydrophilic groups of the nanogels network. The partial substitution of oxidized NC with PE phenolic (hybrid compound) allowed further penetration routes of buffer molecules and increased the swelling ratio from 165.2 to 291.8 [for ONC(1)-PE], 142.5–276.2% [for ONC(2)-PE] and from162.1 to 323.8 [for ONC(3)-PE]. Owing to the high density of hydrophilic groups in the nanocomposites (carboxyl, amide, and hydroxyl groups) led to an increase of swelling capacity.
In vitro release study
The release profile of the CAR drug from Cs-ONCs-PE versus Cs-ONCs was estimated over 24 h in methanol-PBS (pH 7.4) and the effect is illustrated in Fig. 9. It is evident that the CAR drug release profile showed a biphasic release pattern with an initial burst release in the first 5 h followed by a controlled release over a period of 24 h. The initial burst release was attributed to dissolution and diffusion of poorly loaded drug in the system, whereas slow and continuous release was due to diffusion of the drug from the polymer matrix. Although the high swelling ratio of Cs-ONCs-PE over the Cs-ONCs, included the extractive success to control or sustain the in vitro CAR release from gels in complete or partial substitution (hybrid) with high release percentage. The gel of Cs-EP showed lower rate (sustain) of drug release with maximum percentage reached to 62.7% after 29 h. Whereas after 5 h of drug release the Cs-PE achieved 36.2% while in absence of PE the Cs-ONC (1), Cs-ONC (2) and Cs-ONC (3) achieved 50.5, 49.1 and 41.9%, respectively. This illustrated the great benefit of palm extract on the behavior of chitosan as a drug carrier of CAR.
It is interesting to note that gels from hybrid ONCs with PE showed similar or lower rate (more sustainable behavior) to that achieved by Cs-EPC; where at 5 h the in vitro release were 38.0, 33.3 and 35.0%, respectively. These values are lower than those observed in case of extractive free nanogels. Moreover, incorporating the PE with ONCs plays a profound effect on increasing the release % at final tested time (29 h). At 29 h the high drug release of Cs-ONC (1) and Cs-ONC (2) were increased from 54.7 to 60.4 and 55.0% to 58.9%. The reverse trend was noticed in the case of nanocomposite gel from hybrid ONC (3) with PE, whereas the extractive led to decrease the maximum release (57.3%) as compared with the case of Cs-ONC (3) (62.2%). As can be shown, nanogels from ONCs hybridizing with PE are promising sustainable systems which provide 50% release CAR at long time (24 h). This time was greater than reported by literature on using other drug carriers (10–18 h) (Hao et al. 2010; Ashkari et al. 2013; Kumar et al. 2017).
Kinetics and mechanism of drug release
Finding the relation between the drug release and the time was calculated to identify and understand the mechanism of drug release. According to the kinetic parameters in Table 5, the release pattern of the drug from almost PE-containing nanocomposite gels followed the zero-order equation with highest degree of correlation. This means that the release of CAR drug from PE-containing nanocomposite films was only a function of time with constant rate (~ 0.07 h−1) and independent of drug concentration. While the PE-free nanocomposite formulations fitted to Higuchi equation except Cs-ONC (2) fitted zero-order equation.
Conclusion
Sustaining release of drugs is essential to the efficiency of carriers because it leads to continuous release with useful and minimal side effects. In this work we succeeded in prolonging the release of 50% of Carvedilol drug to become 24 h, longer than previously reported, by using. by using formulations Methocel K4M CR, Methocel K15M CR, starch، Avicel PH101, talc and magnesium stearate (~ 10 h) and cellulose ether, lactose, magnesium stearate, crystalline cellulose, polyvinylpyrrolidone (~ 18 h). This was done via loading the drug to novel nanogels prepared from chelating chitosan with hybrid RS-based oxidized nanocelluloses with palm extract.
Data availability
All data generated or analyzed during this study are included in this article.
References
Abdel-Hadi AK, Hosny WM, Basta AH, El-Saied H (1994) Metal chelates with some cellulose derivatives. Pt. II. Polym Plast Technol Eng 33(6):781–791
Ajun W, Yan S, Li G, Huili L (2009) Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr Polym 75:566–574
Ashkari ST, Moghal MMR, Dewan SMR, Amin MN (2013) Development of sustained release matrix tablets of carvedilol and evaluation of polymer effect om in-vitro release pattery. Int J Curr Pharm Clin Res 3(1):18–22
Babu VR, Sairam M, Hosamani KM, Aminabhavi TM (2007) Preparation of sodium alginate-methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr Polym 69:241–250
Basta AH (1998) Preparation, characterization, and properties of paper sheets made from chemically modified wood pulp treated with metal salts. Int J Polym Mater 42(1–2):1–26
Basta AH, El-Saied H (2008) New approach for utilization of cellulose derivatives metal complexes in preparation of durable and permanent-colored papers. Carbohydr Polym 74:301–308
Basta AH, Lotfy VF (2021) Synthesis and evaluating of carbon nanoallotrope-biomacromolecule gel composites as drug delivery systems. J Appl Polym Sci 138(33):50830
Basta AH, Khwaldia K, Aloui H, El-Saied H (2015) Enhancing the performance of carboxymethyl cellulose by chitosan in producing barrier coated paper sheets. Nord Pulp Pap Res J 30(4):617–625
Basta AH, El-Saied H, Baraka AM, Lotfy VF (2016) Comparative evaluation of xerogel-based activated carbons synthesized from aliphatic aldehydes of different chain lengths. Soft Mater 14(4):297–308
Basta AH, El-Saied H, Baraka AM, Lotfy VF (2017) Performance of carbon xerogels in the production of environmentally friendly urea formaldehyde-bagasse composites. Clean Soil Air Water 45(6):1600524
Basta AH, Lotfy VF, Eldewany CY (2021a) Comparison of copper-crosslinked carboxymethyl cellulose versus biopolymer-based hydrogels for controlled release of fertilizer. Polym Plast Technol Mater 60:1884–1897
Basta AH, Lotfy VF, Micky JA, Salem AM (2021b) Liquid crystal behavior of cellulose nanoparticles-ethyl cellulose composites: preparation, characterization, and rheology. J Appl Polym 138(12):50067
Cheng M, Qin Z, Liu Y, Qin Y, Li T, Chen L, Zhua M (2014) Efficient extraction of carboxylated spherical cellulose nanocrystals with narrow distribution through hydrolysis of lyocell fibers by using ammonium persulfate as an oxidant. J Mater Chem A 2:251–258
Coat AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Cong Z, Shi Y, Wang Y, Wang Y, Niu J, Chen N, Xue H (2018) A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int J Biol Macromol 107:855–864
Dafe A, Etemadi H, Dilmaghani A, Mahdavinia GR (2017) Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int J Biol Macromol 97:536–543
Doumeng M, Makhlouf L, Berthet F, Marsan O, Delbé K, Denape J, Chabert FA (2021) Comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym Test 93:106878
El-Saied H, Basta AH, Abdel-Hadi AK, Hosny WM (1994) Metal chelates with some cellulose derivatives. Pt.I. Polym Int 35:27–33
Farokhi M, Mottaghitalab F, Samani S, Shokrgozar MA, Kundu SC, Reis RL, Fatahi Y, Kaplan DL (2018) Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol Adv 36:68–91
Fathy NA, Lotfy VF, Basta AH (2017) Comparative study on the performance of carbon nanotubes prepared from agro-and xerogels as carbon supports. J Anal Appl Pyrol 128:114–120
Filipova I, Fridrihsone V, Cabulis U, Berzins A (2018) Synthesis of nanofibrillated cellulose by combined ammonium persulphate treatment with ultrasound and mechanical processing. Nanomater 8(9):1–11
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
Fu C, Ding C, Sun X, Fu A (2018) Curcumin nanocapsules stabilized by bovine serum albumin-capped gold nanoclusters (BSA-AuNCs) for drug delivery and theranosis. Mater Sci Eng C 87:149–154
Gopi S, Amalraj A, Thomas S (2016) Effective drug delivery system of biopolymers based on nanomaterials and hydrogels. A review. Drug Des 5:1–7
Gouda R, Baishya H, Qing Z (2017) Application of mathematical models in drug release. J Dev Drugs 6:1–8
Habibi Y, Goffin AL, Schiltz N, Duquesne E, Dubois P, Dufresne A (2008) Bionanocomposites based on poly(epsilon-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J Mater Chem 18(41):5002–5010
Hanna DH, Lotfy VF, Basta AH, Saad GR (2020) Comparative evaluation for controlling release of niacin from protein and cellulose–chitosan based hydrogels. Int J Biol Macromol 150:228–237
Hao W-H, Lin T-H, Lu TC (2010) Controlled release formulation, and method for making a controlled release formulation, BR112012007175A2
Ho PH, Lotfy V, Basta A, Trens P (2021) Designing microporous activated carbons from biomass for carbon dioxide adsorption at ambient temperature. A comparison between bagasse and rice by-products. J Clean Prod 294:126–260
Iwamoto S, Kai WH, Isogai A, Iwata T (2009) Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromol 10(9):2571–2576
Jiang F, Han S, Hsieh Y-L (2013) Controlled defibrillation of rice straw cellulose and self-assembly of cellulose nanofibrils into highly crystalline fibrous materials. RSC Adv 3:12366
Jiang H, Wu Y, Han B, Zhang Y (2017) Effect of oxidation time on the properties of cellulose nanocrystals from hybrid poplar residues using the ammonium persulfate. Carbohydr Polym 174:291–298
Juan IM, Alvarez VA, Viviana PC, Analia V (2008) Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 15:149–159
Kabanov AV, Batrakova EV, Nubarov NS, Melik Fedoseev NA, Dorodnich TY, Alakhov VY, Chekhonin VP, Nazarova IR, Kabanov VA (1992) A new class of drug carriers: micelles of poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood in brain. J Control Release 22:141–157
Kajal G, Aniruddha C, Rajan R, Subrata C, Arunabha N (2012) Mathematical modeling of drug release profiles for modified hydrophobic HPMC based hydrogels. Pharmazie 67:147–155
Khalil HE, Alqahtani NK, Darrag HM, Ibrahim H-IM, Emeka PM, Badger-Emeka LI, Matsunami K, Shehata TM, Elsewedy HS (2021) Date palm extract (phoenix dactylifera) PEGylated, development, optimization and cytotoxicity. Plants 10:735
Kumar U, Islam S, Halder S, Rouf AS (2017) Assessment of once daily sustained release hydrophilic matrix tablet of carvedilol. J Pharm Sci 16(1):43–53
Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T (2003) Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release 89:113–125
Lavanya D, Kulkarni P, Dixit M, Raavi PK, Krishna LNV (2011) Source of cellulose derivatives and their applications. Int J Drug Form Res 2:19–38
Levdik I, Inshakov M, Misyurova E, Nikitin V (1967) Study of pulp structure by infrared spectroscopy. Tr Vses Nauch Issled Irst Tsellyul Bum Prom 52:109–111
Lotfy VF, Fathy NA, Basta AH (2018) Novel approach for synthesizing different shapes of carbon nanotubes from rice straw residue. J Env Chem Eng 6(5):6263–6274
Lotfy VF, Hassan SS, Khalf-Alla PA, Basta AH (2020) The role of side chain of amino acid on performance of their conjugates with carboxymethyl cellulose and their Pd(II) complexes as bioactive agents. Int J Polym Mater Polym Biomater 69(1):21–31
MacLaughlin FC, Mumper RJ, Wang J, Tagliaferri JM, Gill I, Hinchcliffe M, Rolland AP (1998) Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J Control Release 56:259–272
Madani H, Wibowo A, Judawisastra H, Nishiyama N, Budhi YW (2022) One-step extraction of cellulose nanocrystals from high lignin biomass through ammonium persulfate oxidation method. Adv Nat Sci Nanosci Nanotechnol 13:015007
Maeda H, Ueda M, Morinaga T, Matsumotog T (1985) Conjugation of poly(styrene-co-maleic acid)derivatives to the antitumor protein-neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem 28:455–461
Maham A, Tang Z, Wu H, Wang J, Lin Y (2009) Protein-based nanomedicine platforms for drug delivery. Small 5:1706–1721
Marwanto M, Maulana MI, Febrianto F, Wistara NJ, Nikmatin S, Masruchin N, Zaini LH, Lee S-H, Kim NH (2021) Characteristics of nanocellulose crystals from balsa and kapok fibers at different ammonium persulfate concentrations. Wood Sci Technol 55:1319–133
Morais JPS, Rosa MDF, De Souza Filho MD, Nascimento SM, Do Nascimento DM, Cassales AR (2013) Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr Polym 91(1):229–235
Nelson M, O’Connor RTJ (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type part I. spectra of lattice types I, 11, I11and of amorphous cellulose. J Appl Polym Sci 8:1325–1341
Olsson RT, Kramer RH, Lopez-Rubio A, Torres-Giner S, Ocio MJ, Lagaron JM (2010) Extraction of microfibrils from bacterial cellulose networks for electrospinning of anisotropic biohybrid fiber yarns. Macromol 43(21):4201–4209
Orrabalis C, Rodríguez D, Pampillo LG, Londoño-Calderón C, Trinidad M, Martínez-García R (2019) Characterization of Nanocellulose obtained from Cereus Forbesii (a South American cactus). Mater Res 22(6):e20190243
Oun AA, Rhim J-W (2018) Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 25:2143–2149
Pal SL, Manna PK, Mohanta GP (2018) Preparation and Physicochemical characterization of carvedilol-poly(lactide-co-glycolic acid_loaded nanoparticles. Int J Life Sci Pharm Res 8(1):79–89
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10
Perteghella S, Crivelli B, Catenacci L, Sorrenti M, Bruni G, Necchi V, Vigani B, Sorlini M, Torre ML, Chlapanidas T (2017) Stem cell-extracellular vesicles as drug delivery systems: new frontiers for silk/curcumin nanoparticles. Int J Pharm 520:86e97
Roman M, Gray DG (2005) Parabolic focal conics in self-assembled solid films of cellulose nanocrystals. Langmuir 21(12):5555–5561
Rosa MF, Medeiros ES, Malmonge JA, Gregorski KS, Wood DF, Mattoso LHC et al (2010) Cellulosenanowhiskers from coconut husk fibers: effect of preparation conditions on their thermal and morphological behavior. Carbohydr Polym 81(1):83–92
Rosli NA, Ahmad I, Abdullah I (2013) Isolation and characterization of cellulose nanofibrils from agave angustifoliafibre. Bioresources 8(2):1893–1908
Salerno H, Verdolotti L, Raucci MG, Saurina J, Domingo C, Lamanna R et al (2018) Hybrid gelatin-based porous materials with a tunable multiscale morphology for tissue engineering and drug delivery. Eur Polym J 99:230–239
Shuai X, Ai H, Nasongkla N, Kim S, Gao J (2004) Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release 98:415–426
Sip S, Paczkowska-Walendowska M, Rosiak N, Miklaszewski A, Grabańska-Martyńska K, Samarzewska K, Cielecka-Piontek J (2021) Chitosan as valuable excipient for oral and topical carvedilol delivery system. Pharmaceuticals 14(8):712
Siqueira G, Bras J, Dufresne A (2010) New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 26(1):402–411
Teixeira EM, Bondancia TJ, Teodoro KBR, Corrêa AC, Marconcini JM, Mattoso LHC (2011) Sugarcane bagasse whiskers: extraction and characterizations. Ind Crops Prod 33(1):63–66
Thakur VK, Thakur MK, Gupta RK (2013) Development of functionalized cellulosic biopolymers by graft copolymerization. Int J Biol Macromol 62:44–51
Thorat MN, Dastager SG (2018) High yield of production of cellulose by Komagataeibacter rhaeticus PG2 strain isolated from pomegranate as a new host. RCS Adv 8:29797
Varshosaz J, Taymouri S, Hamishehkar H (2013) Fabrication of polymeric nanoparticles of poly(ethylene-co-vinyl acetate) coated with chitosan for pulmonary delivery of carvedilol. J Appl Polym Sci https://doi.org/10.1002/APP.39694
Xie M, Li Y, Zhao Z, Chen A, Li J, Li Z, Li G, Lin X (2016) Development of silk fibroin-derived nanofibrous drug delivery system in supercritical CO2. Mater Lett 167:175–178
Xu J, Xu B, Shou D, Xia X, Hu Y (2015) Preparation and evaluation of vancomycin-loaded N-trimethyl chitosan nanoparticles. Polymer 7:1850–1870
Zhao D, Zhuo RX, Cheng SX (2012) Alginate modified nanostructured calcium carbonate with enhanced delivery efficiency for gene and drug delivery. Mol Biosyst 8:753–759
Acknowledgments
This research work was carried out under grant Egypt-China Cooperation Program: funded by Science, Technology, and Innovation Funding Authority with Contact/Agreement No. 43068.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was funded by the Science and Technology Development Fund, Egypt (Grant Number 43068).
Author information
Authors and Affiliations
Contributions
Prof. Dr. A.H.B., Conception and design of study, Investigation, Methodology, Supervision, Drafting the manuscript, Writing—Revising the manuscript critically for important intellectual content. Dr. V.F.L.: Investigation, Methodology, Writing—original draft, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basta, A.H., Lotfy, V.F. The synergistic route for enhancing rice by-product derived nanoparticles in sustained release of bioactive compound. Cellulose 30, 11473–11491 (2023). https://doi.org/10.1007/s10570-023-05560-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05560-7