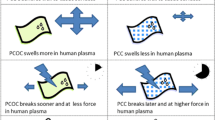
Abstract
Bleeding is one of the most commonly occurring injuries; it can be painful and even life-threatening condition. The hemostats are substances that promote blood clotting and fasten hemostasis. In this paper, we evaluated the hemostatic effect of freeze-dried wound dressings based on equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC) and their mixtures. The wound dressings were investigated for their morphological structure, chemical structure, absorption properties, in vitro hemostasis, cytotoxicity assay and lastly, for in vivo hemostasis. We have found out that adding fibrous CMC into collagen-based hemostatic wound dressings creates a strong synergistic effect, which significantly improves absorption capacity by almost doubling it, as well as supports clotting time. Based on the in vivo studies on partial nephrectomy in rats, the time needed for achieving hemostasis was significantly lower due to the synergy of collagen and CMC. Our materials were compared to the commonly used hemostatic sealing patch on the market (Tachosil) during the in vivo testing, and sample of a mixture of equine collagen and CMC showed better hemostatic efficacy.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The largest organ of the human body is the skin. It is an interference between the organism and the environment, which protects the organism against various threats such as viruses, bacteria, heat, cold, or damage. Being a versatile barrier against the outer environment comes with risks of damage such as abrasions, scratches, or cuts (Xu et al. 2015). These types of wounds might carry a risk of bleeding, which our organism should be able to control by activating platelets and coagulation factors through the whole coagulation cascade process. This process leads to blood clot formation, preventing further blood loss. In some situations where an intense haemorrhage is present, the coagulation cascade might not work fast enough to be able to stop the bleeding. Only in the USA, roughly 60,000 people die every year due to blood loss (Cannon 2018).Significant blood loss is the primary cause of death in combat and the second most common cause of mortality in ordinary trauma centres. In these types of situations, something which might provide help is hemostatics (Peng 2020).
The hemostatics are substances that promote blood clot formation, secure the wound against bacteria, and possibly help us with the process of healing. Hemostatic agents can be divided into several categories according to the mechanism of action by which they accelerate the stopping of bleeding: factor concentrators, procoagulants and mucoadhesives (Kinloch 1980; Wolberg et al. 2012; Behrens et al. 2014; Kaufman et al. 2015; Hong et al. 2019; Jaifu et al. 2019; Li et al. 2019; Peng 2020). The ideal hemostat should have properties such as quick and effective bleeding control, excellent biocompatibility without harmful effects on healing, ease to use, low storage requirements, long shelf-life and ability to remain in place for extended periods of time if needed (Neuffer et al. 2004; Pusateri et al. 2004; Peng 2010) A wide variety of materials are currently used in hemostatic agents, e.g., bovine collagen, oxidized cellulose, carboxymethyl cellulose or chitosan, native chitosan, kaolin, fibrin, thrombin, zeolite or cyanoacrylate(Hughes et al. 2002; Kozen et al. 2008; Martina et al. 2009; Karr et al. 2011; Cho et al. 2013; Moench et al. 2014; Renati et al. 2017).
None of the used materials provides all of the desired properties. The most commonly used hemostats are based on collagen and cellulose derivates. Collagen is one of the most widely used biomaterials. It shows excellent biocompatibility and safety thanks to its biological characteristics—biodegradability and almost no immune system responses. This makes it one of the most used materials in medical applications. The most common application forms are drug delivery systems, fundamental matrices for cell culture systems and tissue engineering (skin replacement, bone substitutes, artificial blood vessels or valves) (Lee et al. 2001; Olsen 2003; Yang et al. 2004; Liu et al. 2008; Wang et al. 2008). From the chemical point of view, collagen is a structural protein with high molecule mass involved in animal tissue. The highest collagen content is located in the skin, tendons, and other connective tissues. It has a fibrous structure made from amino acid chains that form triple helices, and these helices form fibrils that bind together to form collagen fibres (Shoulders and Raines 2009). Collagen-based hemostats support hemostasis through blood cell adsorption, activation and promotion of platelet adhesion and platelets aggregation. These aggregated active platelets further release procoagulant molecules (ADP, Ca2+). They also allow colocalization and activation of coagulation factors on their membrane to augment thrombin production and fibrin formation to accelerate clotting. This is a direct result of contact between collagen and blood. The collagen-based hemostat does not promote biologically active coagulation or does not work in combination with thrombin (Manon-Jensen et al. 2016; Napavichayanun and Aramwit 2017; Rumbaut and Thiagarajan 2010). The risk of using collagen (especially bovine collagen) in the potential health risk regarding bovine spongiform encephalopathy (BSE), other possible diseases and religious reasons (mainly Islam and Judaism) (Nalinanon et al. 2008; Furtado et al. 2022).
Cellulose is an ideal candidate for biomaterial usage thanks to its adjustable mechanical, chemical, and physical properties. The most significant advantage is the easy availability in nature, from which a relatively low product price comes. Another advantage is cellulose properties, which meet three fundamental requirements for biomaterials – biocompatibility, bioactivity and biomechanics (Olsson and Westm 2013; Hickey and Pelling 2019). Carboxymethyl cellulose (CMC) is an anionic, water-soluble cellulose derivative. The solubility of CMC depends on the degree of substitution and the uniformity of the substitution distribution. The most used are sodium or calcium salt of CMC. Thanks to its highly hygroscopic nature, CMC hydrates rapidly, which is very convenient in applications such as hemostatic agents (Ohta et al. 2015; Ergun et al. 2016). CMC is widely used in the food industry for many applications, such as thickeners or stabilizers. In the healthcare industry, it is used in several drug deliveries (time-release, nasal release, gastrointestinal delivery, mucosal delivery), lubricant in eye drops, as a scaffold in tissue engineering, wound healing, and hemostatic agents (Ugwoke et al. 2000; Schwarz 2003; Chen et al. 2010; Aravamudhan et al. 2014). CMC-based hemostatics work on the principle of dissolving in the blood, leading to increased blood viscosity and accelerating the development of clots. Some researchers suggest that CMC acts as a bridge for fibrin polymerization, leading to the formation of thick fibrin formation; on the other hand, other researchers indicate that dissolved CMC activated platelet coagulation.
Using a mixture of these commonly used materials could provide enhanced properties of prepared hemostatics thanks to the fact that there will be two mechanisms of action on fastening the hemostasis. Both help in the improvement of blood clotting in the bleeding wound (Wang et al. 2007; AOSHIMA et al. 2012). Combining collagen and CMC might result in hemostatic agents with superior properties, decreasing the time needed to stop bleeding and thus saving many lives, as we preliminary proved in our previous paper by combining bovine collagen and CMC (Paprskářová et al. 2021). We also proved in one of our previous papers(Babrnáková et al. 2019) that combining collagen with polysaccharides to create wound dressings enhances biocompatibility. The hypothesis of our research was to combine equine or porcine collagen, as a subsidiary type of commonly used bovine collagen, and fibrous carboxymethyl cellulose to form a highly functional freeze-dried hemostatic wound dressing which will have better hemostatic properties than commonly used hemostat on the market—Tachosil. Tachosil is an equine collagen sponge with coated fibrinogen and thrombin on the surface, being cost-ineffective. The usage is mainly to stop minor local internal bleeding (Di Carlo et al. 2011). We have found out that adding fibrous CMC into the equine or porcine collagen creates a synergy effect by enhancing the final hemostatic properties of freeze-dried sponge wound dressings.
Experimental
Materials and methods
The fibrous sodium salt of carboxymethyl cellulose (Holzbecher, Zlíč, Czech Republic) with a degree of substitution DS = 0.602, pH = 4.958 and Mn of 300 kDa (measured by GPC/SEC). Equine and bovine collagen were obtained from Collado s.r.o., Brno, Czech Republic. Phosphate-buffered saline (PBS), Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, trypsin/EDTA, XTT ((2,3-bis-(2- methoxy-4-nitro-5-sulfophenyl)-2 H-tetrazolium-5-carboxanilide) were purchased from Sigma Aldrich (St. Louis, MO, USA). Porcine heparinized blood was obtained from Veterinary Research Institute (Brno, Czech Republic).
Fabrication of wound dressings
Hemostatic wound dressings were prepared either from 1% equine or porcine collagen, 1% CMC or by mixing 1% water-solutions of collagen and CMC. Final solutions were poured into moulds and placed into a lyophilizer (Martin Christ, Epsilon 2 10D LSCPlus, Osterode am Hartz, Germany) and freeze-dried (− 35° C, 15 Pa, 48 h). The grammage of prepared samples was 50 g·m−2. All prepared samples are in Table 1.
Infrared spectroscopy
Fourier-transformed infrared spectroscopy (FTIR) with attenuated total reflectance (ATR-FTIR, Vertex 70/70v, Bruker, Billerica, MA, USA) was performed to characterize the chemical composition of porous foams. Presented ATR-FTIR spectra were taken from averaging 32 scans with a spectral resolution of 4 cm−1. The displayed spectra in the wavenumber range 4000–500 cm−1 were normalized using min–max normalization (OPUS software, Bruker, Billerica, MA, USA). The ATR-FTIR spectra were measured in an evacuated condition on a diamond ATR crystal.
Morphological analysis and porosity
Scanning electron microscopy (SEM) was performed for all foam scaffolds to evaluate their morphology. The surfaces of the intact collagen and CMC scaffolds and the cross sections of these scaffolds were all coated with 15 nm thick gold using Coater Leica EM ACE600 (Wetzlar, Germany). SEM was carried out using Tescan MIRA3-XMU (Brno, Czech Republic) to observe the surface characteristics, pore sizes, and pore distribution of the porous materials. Images were taken in a secondary electron emission mode, scan mode was RESOLUTION, beam density was 10, and high voltage was 5 kV.
The porosity and pore sizes from the images obtained through the SEM analysis were evaluated by ImageJ 2 software.
Absorptive capacity
The samples were cut into pieces of 5 × 5 cm and weighted precisely to the nearest 0.01 g (wdry). The weight of the samples was multiplied by a coefficient of 40. That gave us a weight of a solution which was then slowly added to the sample in a glass container. Physiological saline solution tempered to 37 °C was used as a solution. The container with a wet sample was placed into an incubator at 37 °C for 30 min. Then the sample was mounted with tweezers by a corner and let drip for 30 s. After that, the sample was weighted to the nearest 0.01 g (wet). The absorptive capacity was then calculated according to Eq. (1):
Blood sorption
Enhancing knowledge about how the wound dressings behave in contact with blood and blood absorption was tested. The method was adopted from Ong et al. (2008) and modified. The absorption efficiency of wound dressings was determined in whole heparinized blood. The dressings were weighted (wini) and placed inside a plastic cup, and 1.5mL of blood was added. The cups were closed and put into an incubator at 37 °C for 2 h. After that time, the dressings were dried off any excess blood and weighed (wwet). The capacity was calculated according to Eq. (2):
Clotting time
Clotting time is the time needed for the hemostatic to clot the blood. Testing was done according to Barba et al. 2018. Fresh whole blood was collected from animals, and heparin (10 mg·mL−1) was immediately added to prevent spontaneous clotting. 100 mg or 1 cm3 of the sample was put in an Eppendorf tube, and 1 mL of blood was added. After the addition of blood, the tubes were rotated several times and then every other 30 s. The time was measured until the clot was formed.
Cytotoxicity assay
NIH-3T3 (fibroblast cell line that was isolated from a mouse NIH/Swiss embryo cells, 3-day transfer, inoculum 3×105 cells) were cultured in DMEM (Dulbecco’s Modified Eagle Medium) medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were harvested by trypsinization in a 0.25% solution of trypsin/EDTA in PBS at 80% confluence. The extracted test was used to evaluate the cytotoxic effect of samples. Each material was incubated with complete DMEM culture media at a concentration of 33 mg·mL−1 for 24 h. NIH-3T3 cells were seeded at the concentration of 104 cells/well into a 96-well plate prior to initiating the assay. After 24 h of cell growth, the cell culture media was removed and replaced with the extract media. Cells were then incubated at 37° C and 5% CO2 for 24 h prior to evaluation with XTT assay. XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2 H-tetrazolium-5-carboxanilide) was used according to the manufacturer’s protocols. Briefly, the extract medium was removed from the well plate, and cells were gently washed in PBS buffer solution. Then the 100 µl of fresh DMEM medium and 50 µl XTT (XTT, 1 mg·mL−1 in PBS, pH 7.4) labelling mixture was added per well. Absorbance was measured after 4 h of incubation at 37 °C with a plate reader at 450 nm. The cytotoxic effect of materials was evaluated as the percentage of viable cells. As a positive control the empty plastic well plate was used.
In vivo partial nephrectomy
The whole method was provided according to the published procedure (Paprskářová et al. 2021). Sixty male Wistar laboratory rats (AnLab, Czech Republic) weighing 265 ± 62 g were allocated into six groups of ten animals at random. I.m. administration of a blend of tiletamine and zolazepam was used to anaesthetize these animals (Zoletil 100, Virbac 5 1., France). It was given a dose of 65 mg·kg−1. The peritoneum was then surgically opened, and a partial nephrectomy of the caudal pole of the left kidney was performed. Immediately after this action, 1 cm2 pieces of hemostatics were applied to the incisions until achieving hemostasis. The time needed for hemostasis was measured. The hemostatics were left at the wound after hemostasis was achieved. The wounded kidney was returned to the peritoneal cavity. The peritoneum and skin were sutured.
Ethic
All in vivo testing was done at Veterinary and Pharmaceutical University (currently Faculty of Pharmacy at Masaryk University, Brno) in accordance with the guidelines of SUKL (State Institute for Drug Control) and the Ministry of Health of the Czech Republic. The Scientific Committee for the Protection of Animals at the university approved the in vivo testing. After approval, a partial nephrectomy model in rats was carried out.
Statistical analysis
Origin lab software was used to carry out statistical analysis of obtained results. All of the data was evaluated using Tukey’s test, using five samples of each type (ten samples for in vivo testing), which uses pairwise post-hocptesting. The level of significance was set at 0.001, 0.01 and 0.05. Data from cytotoxicity testing were analyzed using Student’s t-test with a confidence level of 95%. The data was presented as mean ± standard error (n = 3).
Results
Composition evaluation
The chemical composition of freeze-dried foams was evaluated by the ATR-FTIR method in a dry state. Figure 1a shows the ATR-FTIR spectra of porcine collagen and equine collagen and, in combination with fibrous CMC, Por+CMC and Eq+CMC. There is a characteristic absorption related to the O–H group and the N–H stretching bonds, including collagen amide A bond at 3319 cm−1 and amide B at 2924−2854 cm−1, more clearly seen in equine collagen and Por+ CMC. In all collagen samples, there are typical characteristic absorptions attributed to the stretching vibrations of the C=O related to amide I at 1745–1652 cm−1, amide II with vibrations of the N–H bands and the vibrations of the C–N at 1564–1454 cm−1, and amide III with vibrations of the C–N stretching, the N–H bending, and the vibrations of the CH3 groups at 1235 cm−1. Figure 1b shows the characteristic wavenumber range of CMC with dominant vibration of a carbon-oxygen single bond, denoted as C–O at 1061 cm−1. The rest absorption spectra are in more detail depicted in Fig. 1a. There is low intensity vibration at 1061 cm−1 in Por+CMC and Eq+CMC samples, confirming the CMC addition in the samples.
Sponge morphology and porosity
Morphological SEM analysis revealed that the collagen-based and CMC sponges obtained through the lyophilization method showed highly interconnective porous structures. The analysis also showed that the surface of the prepared samples was rather rough, and the roughness of the foams was considerably similar to each other. However, the pure CMC scaffold showed different properties. The surface of the CMC foam was found to be smoother and revealed a compact microporous structure (Figs. 2).
The pores size measurements showed that the overall average pore size (Fig. 3) of all the prepared collagen-based and CMC scaffolds was 97.16 μm and the foams showed a well-developed macroporous structure with pore sizes ranging from 42.03 to 161.71 μm. Individually the greatest porosity was observed in the Por+CMC scaffolds with an average pore size of 161.71 ± 116.38 μm with high porosity and pore distribution, making this macroporous matrix ideal for cell migration and ion transport. The Eq+CMC has an average pore size of 95.76 ± 88.67 μm. The pure equine collagen (average pore size of 70.93 ± 61.08 μm), as well as the pure CMC (average pore size of 72.76 ± 113.12 μm) scaffolds were found to have the smallest porosity with a small range of pore distribution. The porcine collagen has a pore size of 138.68 ± 135.19 μm.
Box-plot graph of pore size distribution in equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC), and mixtures of equine collagen + fibrous CMC (Eq+CMC) and porcine collagen + fibrous CMC (Por+CMC). p values reaching statistical significance (p < 0.001) were marked ***between all of the compared samples
Absorptive capacity
The absorptive capacity was performed according to ČSN EN 13726-1. The sample of fibrous CMC has an absorption capacity of 37.08 ± 0.13, which is the highest of all samples. The equine collagen and porcine collagen had an absorption capacity of 17.7 ± 0.98 and 9.15 ± 1.45, respectively. The combined sponges of Eq+CMC and Por+CMC exhibited a value of 26.41 ± 0.7 and 15.65 ± 2.55. The obtained results clearly show that by adding the CMC into the collagen wound dressing, there is a significant improvement in absorption properties when compared to collagens alone. Porcine collagen alone has very low absorption, but when CMC is added, the capacity is almost doubled. The synergy of collagen and fibrous CMC is clearly visible (Figs. 4, 5).
The absorption capacity according to ČSN EN 13726-1 of measured samples. The tested samples were as follows: equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC), and mixtures of equine collagen with CMC (Eq+ CMC) and porcine collagen with CMC (Por+CMC). p values reaching statistical significance (p < 0.001) were marked *** between all of the compared samples
Blood locking in the inner structure of the sponges
To test the ability of samples to absorb and lock blood inside its structure, we lay a sample on the blood-soaked sponge and watched the sample absorption and the ability to lock the liquid inside its structure without leaking by removing the dressings from the sponge and holding it with tweezers. The results in Fig. 6 showed that the samples with CMC allow higher sorption and support the mechanical properties of the wet sample. The samples are stable and can hold a large amount of blood in their inner structure without any leak. Image f shows that we can pick up the wound dressings with tweezers, and in picture g it is clearly visible that the sample is holding the blood inside of its structure and still maintains mechanical properties.
Schematic pictures of blood locking inside the sponge wound dressing’s structure; first contact with blood (a), the start of blood absorption (b), the diameter of the sample is almost absorbed (c), penetration of the blood (d) and (e). Complete penetration of the blood with maintained mechanical properties (f) and (g)
Blood sorption and clotting time
The sorption of blood might differ from the absorption capacity of saline solution thanks to the interaction between the wound dressing and blood proteins, clotting factors, and other blood components. The CMC showed the best sorption properties as well as absorptive capacity testing. The blood sorption is 36.30 ± 3.36, which is far better sorption properties than the collagens dressing. Equine collagen and porcine collagen have almost similar sorption properties, with 11.80 ± 0.92 and 11.39 ± 1.20 values, respectively. The mixtures have slightly elevated sorption, but the difference between pure collagens and the mixture is not as significant as in absorption capacity testing. The mixture of Eq+CMC and Por+CMC showed the sorption of 15.45 ± 1.21 and 13.44 ± 0.42, respectively. The CMC create a sort of gel in contact with the blood and locks a large amount of it inside its structure. The porcine collagen shows comparable sorption in absorption capacity with saline solution, whereas the equine collagen has lower sorption properties with blood. Minor improvement in sorption can be seen when the collagen is enhanced with CMC (Figs. 7, 8).
Blood sorption testing. The tested samples were as follows: equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC), and a mixture of equine collagen with CMC (Eq+CMC), and porcine collagen with CMC (Por+CMC). p values reaching statistical significance (P < 0.001) were marked *** between samples of collagens and CMC and between samples of mixtures of collagens with CMC compared to CMC
Clotting time is an in vitro hemostatic testing which simulates blood clotting by mimicking the interaction of hemostatic wound dressing and whole blood. The results shown in Fig. 9 clearly show that the CMC has superior clotting properties in comparison to other tested samples. The clotting time of CMC is 11.67 ± 2.08s. Equine collagen clotting time is 32.0±5.30 s and of Porcine collagen 50.34 ± 2.52 s. The clotting time of collagens and CMC mixture is lower than collagens alone. The Eq+CMC has 20.67 ± 3.06 s and Por+CMC has 33.0 ± 5.0 s.
a Results of clotting time testing shows the time needed for blood clot formation. The tested samples were as follows: equine collagen, Porcine collagen, fibrous carboxymethyl cellulose (CMC), and a mixture of equine collagen with CMC (Eq +CMC) and Porcine collagen with CMC (Por+CMC). p values reaching statistical significance (P < 0.001) were marked *** between samples of equine collagen and porcine collagen compared to CMC; porcine collagen to a mixture of equine collagen with CMC and between a mixture of porcine collagen with CMC and CMC. p values reaching statistical significance (p < 0.01) were marked ** between equine collagen and porcine collagen and between porcine collagen and a mixture of porcine collagen with CMC. p values reaching statistical significance (p < 0.05) were marked * between equine collagen and a mixture of equine collagen with CMC and a mixture of porcine collagen with CMC and a mixture of equine collagen and CMC. Picture b shows samples during clotting time testing
Cytotoxicity of prepared foams
The application of the hemostatic material requires a time in seconds, and therefore we chose a 24-h extraction test as the maximum for the evaluation of cytotoxicity as sufficient. The extraction test corresponds with the cytotoxic effect of any leachable (by)products from tested samples. The results showed the highest viability, up to 80%, for porcine collagen extract, while the cell viability in equine collagen extract showed the lowest viability, slightly over 20%. The synergy of either Eq or Por with CMC, as well as pure CMC, have shown similarities in cell viability between 40 and 60%. However, the mixture of equine collagen with CMC showed the best balance between pure CMC and tested collagens and reached almost 55% viability compared with the plastic control (Fig. 10). The pH values of the extracts (Table 2), were measured to help understand cytotoxicity testing and obtained additional data.
Cytotoxicity evaluation of samples using XTT-based extract test. The tested samples were as follows: equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC), and a mixture of equine collagen with CMC (Eq+CMC), and porcine collagen with CMC (Por+CMC). p values reaching statistical significance (p < 0.05; n = 3) between control (plastic) and all samples are marked as * and ** indicates p < 0.05 between collagen samples and mixtures, and *** indicates p < 0.05 between CMC and mixtures
In vivo hemostasis evaluation by partial nephrectomy
To determine the hemostatic properties in a living organism, in vivo partial nephrectomy testing was performed. Equine collagen, porcine collagen, CMC and their mixtures were tested alongside the Tachosil, which is a commonly used hemostatic on the market, based on human fibrinogen, as a comparison. The results are shown in Fig. 11, where we can see how much time was needed in order to stop bleeding. The control sample, Tachosil, stopped the bleeding after 7.44 ± 1.59 s, CMC after 6.9 ± 3.11 s, equine collagen in 25.6 ± 5.52 s and Porcine collagen in 44.5 ± 16.37 s. The mixtures showed improved properties than pure collagens; Eq+CMC stopped the bleeding fastest at 2.5 ± 0.97 s and Por+CMC at the time, similar to Tachosil (8.2 ± 3.65 s). There is a significant difference between samples of pure collagen and mixtures of collagens with CMC, confirming that the addition of CMC into the wound dressings contributes to faster hemostasis (Figs. 12).
a Results of the in vivo hemostasis test in partial nephrectomy in rats. The results are shown how long it takes to stop the bleeding. The tested samples were as follows: equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC), and a mixture of equine collagen with CMC (Eq+CMC) and porcine collagen with CMC (Por+CMC). p values reaching statistical significance (p < 0.001) were marked *** between equine collagen and porcine with all of the other samples. Picture b photo of rat kidney during partial nephrectomy
The consumption of the hemostats is an important parameter as it lowers the price of the used product when you need to use fewer amounts of hemostatic to achieve the same hemostatic effect. The mixtures report lesser consumption than collagens or CMC alone. The consumption of equine collagen was 2.5 ± 0.53 cm2, 3.1 ± 0.74 cm2 of porcine collagen, and CMC consumption was 2.9 ± 0.88 cm2. The consumption of mixtures was lower than pure materials; the consumption of equine collagen with CMC (1.3 ± 0.48 cm2) was similar to Tachosil (1.33 ± 0.5 cm2).
Evaluation of usage of wound dressings in terms of hemostatic being used on the wound to achieve hemostasis. The tested samples were as follows: equine collagen, porcine collagen, fibrous carboxymethyl cellulose (CMC), and a mixture of equine collagen with CMC (Eq+CMC) and porcine collagen with CMC (Por+CMC). p values reaching statistical significance (p < 0.001) were marked *** between samples of porcine collagen and a mixture of equine collagen with CMC, porcine collagen and Tachosil, CMC and a mixture of equine collagen with CMC and CMC with Tachosil. p values reaching statistical significance (p < 0.01) were marked ** between equine collagen and a mixture of equine collagen with CMC, equine collagen and Tachosil, and porcine collagen and a mixture of porcine collagen and CMC. p values reaching statistical significance (p < 0.05) were marked * between CMC and a mixture of porcine collagen with CMC.
Discussion
Hemostatic wound dressings are a versatile aid in stopping bleeding and achieving hemostasis of the cause of the bleeding. The wound dressing might come in various forms and application designs. The collagen-based and carboxymethyl cellulose-based hemostatics are nowadays commonly used in medicine to stop bleeding. The idea of our research was to combine equine or porcine collagen, as a subsidiary type of collagen to mostly used bovine collagen, and fibrous carboxymethyl cellulose to form a highly functional freeze-dried hemostatic wound dressing which will have better hemostatic properties than commonly used hemostatic on the market (Tachosil).
Overall characterization experiments were conducted to support the hypothesis that using mixtures of collagens with fibrous CMC creates hemostatic wound dressings with improved properties, which could lead to faster hemostasis and, thus, lower blood loss. SEM morphological analysis showed that the freeze-dried sponges have a highly porous structure with interconnected pores of average size 42.03-161.71 μm. The samples consisting of equine collagen and CMC, which showed the best in vivo properties, have a pore size of 95.76±88.67 mm, which might imply that this is the right pore size for interaction with blood. The size of pores supports the finding of Shou et al. (2020), who made chitosan/DOPA-based hydrogel with a pores size range of 100 to 150 μm. The porous structure is an important parameter that allows liquid/blood to be absorbed into the structure and interact with as much of the wound dressing material as possible. The high inner surface allows the collagen and CMC to interact with the coagulation cascade faster and thus increase the cascade speed of action. The absorption capacity is an important characteristic of primary wound dressings, as it implies the ability to concentrate clotting factors and thus improve the hemostasis speed. (Huang et al. 2019). For hemostatic wound dressing, it is important to find the perfect ratio of absorption. When absorption is too high, and swelling of the wound dressing occurs, it can potentially create a localized pressure that is undesirable (Ranjbar et al. 2021). On the other hand, when the absorption is low, the concentration of clotting factor might not occur or can happen at a slower pace, resulting in uncontrolled bleeding. The best overall abortion capacity was measured for the fibrous CMC, which in contact with saline solution, creates a transparent rigid sort of hydrogel that locks a large amount of liquid into its structure but maintains excellent mechanical properties. Equine and porcine collagen did not perform so well when tested alone, but the mixture of them with fibrous CMC strongly enhanced their absorption properties and made them hold a large amount of saline solution, which might help the overall hemostatic activity, as it will be more likely to concentrate the clotting factors. After the absorption capacity according to Czech CSN EN 13726-1, the blood sorption was also tested to assess how the wound dressings behave in interaction with blood. The fibrous CMC also showed identical sorption as when it interacted with the saline solution. The CMC was able to hold a large amount of blood in its structure and lock it inside of it and did not lose shape or mechanical properties. This also allows wound dressings to absorb exudate from the wound and keep the wound clean. The equine collagen performs worse in blood than in saline solution, which might be caused by the fact that the whole blood started to clot on the surface of the collagen wound dressing, and thus it prevented the penetration of the aqueous part of the blood deeper into the sample. On the other hand, porcine collagen has a slightly elevated value, which might indicate that the interaction between porcine collagen and blood is not as strong as in the case of equine collagen. This might be due to a protein interaction between the wound dressing and blood. The addition of CMC to collagen has again improved the effect on the sorption. These results lead to the conclusion that CMC enhances the sorption properties of collagen and might be able to promote hemostasis in synergy with collagens, as with the in vitro hemostatic testing, a clotting time test was performed. The results showed superior clotting properties of fibrous CMC, but CMC does not necessarily create a clot due to its hemostatic properties but because of the ability to absorb a large amount of liquid and create a sort of hydrogel that acts like a clot. This creates a great synergy in collagen mixtures when there are two mechanisms of action – creating a clot made from CMC and then collagen interacting with blood proteins and supporting platelet adhesion and complete activation of the coagulation cascade. This property could be beneficial for hemostatics function in our mixture of CMC with collagen, as it could quickly seal the wound, and then the collagen component could engage and accelerate the body’s response to bleeding and significantly reduce the time needed for hemostasis. Porcine collagen showed poor clotting time, and this could be due to the fact that the porcine collagen could have contained pig fat residues, whereas the horse is a lean animal, and the obtained collagen might have a higher purity of proteins. If we compare the results of the clotting time with those of Schroeder et al. (2021), our results show the lower time needed for hemostasis.
Collagens and CMC are known for their biocompatibility and low cytotoxicity. Our results from cytotoxicity testing of the extracts from the wound dressing showed higher toxicity of the extracts compared to the plastic control. This result is quite surprising and might be caused by the low pH of collagen extract. The value of the pH level of extracts could play a vital role in the cytotoxicity testing of wound dressings, as the cell viability is affected by pH (Kruse et al. 2017). Porcine collagen has higher pH than equine, which correspond with obtained results. The low level of pH could also affect the in vivo testing because the lower pH can enhance blood clot formation (Gissel et al. 2016). The lower pH in the wound caused by the wound dressings might even help with later healing of the wound, as a low pH level below four is believed to improve healing properties. (Milne and Connolly 2014; Percival et al. 2014). This corresponds with our in vivo testing, where the samples with lower pH level act as the better hemostat. For the final assessment of created hemostatic wound dressings, the in vivo partial nephrectomy was performed in rats to specify the hemostatic properties of the wound dressings in the bleeding wound. Testing validated our assumptions from the in vitro, where we stated that porcine collagen has worse properties than equine collagen. CMC works as a great hemostatic material and, in a mixture with equine collagen, even better. Collagens alone did not report as great properties as Tachosil, but in a mixture with CMC, it creates a highly functional hemostatic. The mixture of equine collagen and fibrous CMC collagen has hemostatic properties similar to Tachosil, which makes it a great protentional candidate for further research and testing. Using wound dressings based on equine collagen and CMC might provide the same hemostatic properties as Tachosil but at a lower price, thanks to the absence of expensive proteins such as thrombin and fibrinogen, which could make it more affordable and available. The interesting fact is that the consumption of the mixture of equine collagen and CMC was lower than the average of the individual substances. It might be caused by some synergy and highly functional interactions between them, where the CMC creates the hydrogel structure, seals the wound and allows the collagen to actively interact with clotting factors and blood proteins and enhances the hemostasis. Hemostatic tests in rats were also conducted by Goncharuk et al. (2021) when they tested polyvinyl formal sponges on rats’ livers, they didn’t measure the time needed for achieving hemostasis, but they measured blood loss. (Zheng et al. 2023) tested W-HAP-PVA aerogel (composed of ultralong hydroxyapatite nanowires and poly(vinyl alcohol) on rats’ liver, and in rabbit femoral artery injury and when compared, our wound dressings can achieve faster hemostasis time. All the main results show great potential in the addition of fibrous CMC into collagen-based hemostatic wound dressings. As we look at the obtained results of absorption capacity and blood sorption, the addition of fibrous CMC improved the absorption properties of used collagen and led to a more highly functional wound dressing. The hemostatic tests consisting of clotting time and partial nephrectomy in rats support our hypothesis that the fibrous CMC does enhance the properties of collagen. When we look at the results, the enhancement of hemostasis is significant, and the mixtures of equine collagen and porcine collagen with the addition of fibrous CMC have hemostatic properties comparable to the Tachosil. The development of effective hemostatic materials is a complex and challenging task. Collagen and CMC are two natural polymers that have shown great promise for hemostatic applications. The use of collagen-CMC composites has been shown to be an effective strategy for enhancing hemostasis. However, there are still several challenges that need to be addressed in order to develop these materials for clinical use. These include optimizing the composition and structure of the composite materials, improving their mechanical properties, and ensuring their safety and efficacy in humans.
Conclusion
This conducted study showed that the different types of collagens and fibrous CMC can be combined to create composite hemostatic wound dressing materials that have improved hemostatic properties compared to the individual components. The addition of CMC to collagen-based materials can increase their water/blood absorption capacity, which enhances their ability to form a stable clot and stop bleeding. Collagen-CMC composites have been shown to be effective in promoting hemostasis in vitro and also in vivo. Using the composite of collagen and CMC reduces the number of hemostatic sponges to be applied to the bleeding wound, which also could make the treatment cheaper. Collagen and CMC-based hemostatic materials have shown great potential for use in the treatment of bleeding wounds. Further research is needed to optimize the composition and structure of these materials and to ensure their safety and efficacy in humans. The development of effective hemostatic materials based on collagen and CMC has the potential to improve patient outcomes and reduce healthcare costs.
Data availability
The authors confirm that all relevant data are included in the paper, and the raw data are available upon request from the corresponding author.
Change history
03 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10570-023-05571-4
References
Aoshima M, Tanabe K, Kohno I, Jo Y, Takahashi K, Sugo T, Matsuda M (2012) Hemostatic mechanisms of a soluble fraction of plant-derived sodium carboxymethyl cellulose. J Thromb Haemost 23(4):387–398. https://doi.org/10.2491/jjsth.23.387
Aravamudhan A, Ramos DM, Nada AA, Kumbar SG (2014) Natural polymers. Nat Synth Biomed Polym 67:89
Babrnáková J, Pavliňáková V, Brtníková J, Sedláček P, Prosecká E, Rampichová M, Filová E, Hearnden V, Vojtová L (2019) Synergistic effect of bovine platelet lysate and various polysaccharides on the biological properties of collagen-based scaffolds for tissue engineering: Scaffold preparation, chemo-physical characterization, in vitro and ex ovo evaluation. Mater Sci Eng C 100:236–246. https://doi.org/10.1016/j.msec.2019.02.092
Barba BJD, Aranilla CT, Relleve LS, Cruz VRC, Vista JR, Abad LV (2018) Hemostatic granules and dressing prepared from formulations of carboxymethyl cellulose, kappa-carrageenan and polyethylene oxide crosslinked by gamma radiation. Radiat Phys Chem 144:180–188. https://doi.org/10.1016/j.radphyschem.2017.08.009
Behrens AM, Sikorski MJ, Kofinas P (2014) Hemostatic strategies for traumatic and surgical bleeding. J Biomed Mater Res A 102(11):4182–4194. https://doi.org/10.1002/jbm.a.35052
Cannon JW (2018) Hemorrhagic shock. N Engl J Med 378(4):370–379. https://doi.org/10.1056/NEJMra1705649
Chen R-N, Ho H-O, Yu C-Y, Sheu M-T (2010) Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Eur J Pharm Sci 39(1–3):82–89. https://doi.org/10.1016/j.ejps.2009.10.015
Cho K, Shin S, Lee J, Kim J, Koo S, Kim Y, Kim M, Roh H (2013) The efficacy of cutanplast nasal packing after endoscopic sinus surgery: a prospective, randomized, controlled trial. Laryngoscope 123(3):564–568. https://doi.org/10.1002/lary.23643
Ergun R, Guo J, Huebner-Keese B (2016) Cellulose. Encyclopedia of food and health. Elsevier, Amsterdam, pp 694–702
Furtado M, Chen L, Chen Z, Chen A, Cui W (2022) Development of fish collagen in tissue regeneration and drug delivery. Eng Regen 3(3):217–231. https://doi.org/10.1016/j.engreg.2022.05.002
Gissel M, Brummel-Ziedins KE, Butenas S, Pusateri AE, Mann KG, Orfeo T (2016) Effects of an acidic environment on coagulation dynamics. J Thromb Haemost 14(10):2001–2010. https://doi.org/10.1111/jth.13418
Goncharuk O, Korotych O, Samchenko Yu, Kernosenko L, Kravchenko A, Shtanova L, Tsуmbalуuk O, Poltoratska T, Pasmurtseva N, Mamyshev I et al (2021) Hemostatic dressings based on poly(vinyl formal) sponges. Mater Sci Eng C 129:112363. https://doi.org/10.1016/j.msec.2021.112363
Hickey RJ, Pelling AE (2019) Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol 7:45. https://doi.org/10.3389/fbioe.2019.00045
Hong Y, Zhou F, Hua Y, Zhang X, Ni C, Pan D, Zhang Y, Jiang D, Yang L, Lin Q et al (2019) A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat Commun 10(1):2060. https://doi.org/10.1038/s41467-019-10004-7
Huang H, Chen H, Wang X, Qiu F, Liu H, Lu J, Tong L, Yang Y, Wang X, Wu H (2019) Degradable and bioadhesive alginate-based composites: an effective hemostatic agent. ACS Biomater Sci Eng 5(10):5498–5505. https://doi.org/10.1021/acsbiomaterials.9b01120
Hughes MA, Yang Y, Cherry GW (2002) Effect of traumacel P on the growth of human dermal fibroblasts in vitro. J Wound Care 11(4):149–154. https://doi.org/10.12968/jowc.2002.11.4.26392
Jaifu J, Thunsiri K, Udomsom S, Boonyawan D, Wattanutchariya W (2019) Blood absorption improvement of a naturally derived hemostatic agent by atmospheric pressure plasma jet. Mater Today Proc 17:2088–2096. https://doi.org/10.1016/j.matpr.2019.06.258
Karr JC, Taddei AR, Picchietti S, Gambellini G, Fausto AM, Giorgi F (2011) A morphological and biochemical analysis comparative study of the collagen products biopad, promogram, puracol, and colactive. Adv Skin Wound Care 24(5):208–216. https://doi.org/10.1097/01.ASW.0000397897.18003.ce
Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ et al (2015) Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 162(3):205–213. https://doi.org/10.7326/M14-1589
Kinloch AJ (1980) The science of adhesion: part 1 surface and interfacial aspects. J Mater Sci 15(9):2141–2166. https://doi.org/10.1007/BF00552302
Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS (2008) An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med 15(1):74–81. https://doi.org/10.1111/j.1553-2712.2007.00009.x
Kruse CR, Singh M, Targosinski S, Sinha I, Sørensen JA, Eriksson E, Nuutila K (2017) The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: in vitro and in vivo study. Wound Repair Regen 25(2):260–269. https://doi.org/10.1111/wrr.12526
Lee CH, Singla A, Lee Y (2001) Biomedical applications of collagen. Int J Pharm 221(1–2):1–22. https://doi.org/10.1016/S0378-5173(01)00691-3
Li Z, Milionis A, Zheng Y, Yee M, Codispoti L, Tan F, Poulikakos D, Yap CH (2019) Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat Commun 10(1):5562. https://doi.org/10.1038/s41467-019-13512-8
Liu W, Merrett K, Griffith M, Fagerholm P, Dravida S, Heyne B, Scaiano JC, Watsky MA, Shinozaki N, Lagali N et al (2008) Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 29(9):1147–1158. https://doi.org/10.1016/j.biomaterials.2007.11.011
Manon-Jensen T, Kjeld NG, Karsdal MA (2016) Collagen‐mediated hemostasis. J Thromb Haemost 14(3):438–448. https://doi.org/10.1111/jth.13249
Martina B, Kateřina K, Miloslava R, Jan G, Ruta M (2009) Oxycellulose: significant characteristics in relation to its pharmaceutical and medical applications. Adv Polym Technol 28(3):199–208. https://doi.org/10.1002/adv.20161
Milne SD, Connolly P (2014) The influence of different dressings on the pH of the wound environment. J Wound Care 23(2):53–57. https://doi.org/10.12968/jowc.2014.23.2.53
Moench C, Mihaljevic AL, Hermanutz V, Thasler WE, Suna K, Diener MK, Seehofer D, Mischinger HJ, Jansen-Winkeln B, Knaebel HP et al (2014) Randomized controlled multicenter trial on the effectiveness of the collagen hemostat Sangustop® compared with a carrier-bound fibrin sealant during liver resection (ESSCALIVER study, NCT00918619). Langenbecks Arch Surg 399(6):725–733. https://doi.org/10.1007/s00423-014-1203-9
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2008) Tuna pepsin: characteristics and its use for collagen extraction from the skin of Threadfin Bream (Nemipterus spp). J Food Sci 73(5):C413–C419. https://doi.org/10.1111/j.1750-3841.2008.00777.x
Napavichayanun S, Aramwit P (2017) Effect of animal products and extracts on wound healing promotion in topical applications: a review. J Biomater Sci Polym Ed 28(8):703–729. https://doi.org/10.1080/09205063.2017.1301772
Neuffer MC, McDivitt J, Rose D, King K, Cloonan CC, Vayer JS (2004) Hemostatic dressings for the first responder. Rev Mil Med 169(9):716–720. https://doi.org/10.7205/MILMED.169.9.716
Ohta S, Nishiyama T, Sakoda M, Machioka K, Fuke M, Ichimura S, Inagaki F, Shimizu A, Hasegawa K, Kokudo N et al (2015) Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent. J Biosci Bioeng 119(6):718–723. https://doi.org/10.1016/j.jbiosc.2014.10.026
Olsen D (2003) Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev 55(12):1547–1567. https://doi.org/10.1016/j.addr.2003.08.008
Olsson C, Westm G (2013) Direct dissolution of cellulose: background, means and applications. Cellulose-fundamental aspects. InTech
Ong S-Y, Wu J, Moochhala SM, Tan M-H, Lu J (2008) Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 29(32):4323–4332. https://doi.org/10.1016/j.biomaterials.2008.07.034
Paprskářová A, Suchý P, Chalupová M, Michlovská L, Klusáková J, Sopuch T, Vojtová L (2021) Evaluation and comparison of structurally different cellulose-based hemostatic agents in a rat kidney model. Cellulose 28(14):9369–9382. https://doi.org/10.1007/s10570-021-04104-1
Peng T (2010) Biomaterials for hemorrhage control. Trends Biomater Artif Organs 24(1):27–68
Peng HT (2020) Hemostatic agents for prehospital hemorrhage control: a narrative review. Mil Med Res 7(1):13. https://doi.org/10.1186/s40779-020-00241-z
Percival SL, McCarty S, Hunt JA, Woods EJ (2014) The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen 22(2):174–186. https://doi.org/10.1111/wrr.12125
Pusateri AE, Delgado A, Dick EJ, Martinez RS, Holcomb JB, Ryan KL (2004) Application of a granular mineral-based hemostatic agent (QuikClot) to reduce blood loss after grade V liver injury in swine. J TRAUMA 57(3):555–562. https://doi.org/10.1097/01.TA.0000136155.97758.CD
Ranjbar J, Koosha M, Chi H, Ghasemi A, Zare F, Abdollahifar MA, Darvishi M, Li T (2021) Novel chitosan/gelatin/oxidized cellulose sponges as absorbable hemostatic agents. Cellulose 28(6):3663–3675. https://doi.org/10.1007/s10570-021-03699-9
Renati S, Kaur S, Kresak JL, Wicklund M, Malaty I (2017) Granulomatous meningitis secondary to Avitene (microfibrillar collagen). Neurol Clin Pract 7(5):384–386. https://doi.org/10.1212/CPJ.0000000000000305
Rumbaut RE, Thiagarajan P (2010) Platelet-vessel wall interactions in hemostasis and thrombosis. Colloquium Ser Integr Syst Physiology Molecule Function 2(1):1–75. https://doi.org/10.4199/C00007ED1V01Y201002ISP004
Schroeder JA, Kuether EA, Fang J, Jing W, Weiler H, Wilcox DA, Montgomery RR, Shi Q (2021) Thromboelastometry assessment of hemostatic properties in various murine models with coagulopathy and the effect of factor VIII therapeutics. J Thromb Haemost 19(10):2417–2427. https://doi.org/10.1111/jth.15456
Schwarz UI (2003) Clinical relevance of genetic polymorphisms in the human CYP2C9 gene: CYP2C9 polymorphisms: implementation for drug therapy. Eur J Clin Invest 33:23–30. https://doi.org/10.1046/j.1365-2362.33.s2.6.x
Shou Y, Zhang J, Yan S, Xia P, Xu P, Li G, Zhang K, Yin J (2020) Thermoresponsive Chitosan/DOPA-based hydrogel as an injectable therapy approach for tissue-adhesion and hemostasis. ACS Biomater Sci Eng 6(6):3619–3629. https://doi.org/10.1021/acsbiomaterials.0c00545
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78(1):929–958. https://doi.org/10.1146/annurev.biochem.77.032207.120833
Toro A, Mannino M, Reale G, Di Carlo I (2011) TachoSil use in abdominal surgery: a review. J Blood Med 31(2):31–36. https://doi.org/10.2147/JBM.S13061
Ugwoke MI, Agu RU, Vanbilloen H, Baetens J, Augustijns P, Verbeke N, Mortelmans L, Verbruggen A, Kinget R, Bormans G (2000) Scintigraphic evaluation in rabbits of nasal drug delivery systems based on carbopol 971p® and carboxymethylcellulose. J Control Release 68(2):207–214. https://doi.org/10.1016/S0168-3659(00)00258-3
Wang Y, Cui FZ, Hu K, Zhu XD, Fan DD (2008) Bone regeneration by using scaffold based on mineralized recombinant collagen. J Biomed Mater Res B Appl Biomater 86B(1):29–35. https://doi.org/10.1002/jbm.b.30984
Wang H, Wang C, Yao J, Liu K (2007) Study on hemostatic mechanism of fully soluble hemostatic fiber. Blood Coagul Fibrinolysis 18(6):555–558. https://doi.org/10.1097/MBC.0b013e3281eec966
Wolberg AS, Aleman MM, Leiderman K, Machlus KR (2012) Procoagulant activity in hemostasis and thrombosis. Anesth Analg 114(2):275–285. https://doi.org/10.1213/ANE.0b013e31823a088c
Xu R, Luo G, Xia H, He W, Zhao J, Liu B, Tan J, Zhou J, Liu D, Wang Y et al (2015) Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 40:1–11. https://doi.org/10.1016/j.biomaterials.2014.10.077
Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW (2004) The application of recombinant human collagen in tissue engineering. BioDrugs 18(2):103–119. https://doi.org/10.2165/00063030-200418020-00004
Zheng Y, Wu J, Zhu Y, Wu C (2023) Inorganic-based biomaterials for rapid hemostasis and wound healing. Chem Sci 14(1):29–53. https://doi.org/10.1039/D2SC04962G
Acknowledgement
CzechNanoLab project LM2023051 funded by MEYS CR is gratefully acknowledged for the financial support of the measurements/sample fabrication at CEITEC Nano Research Infrastructure.
Funding
Open access publishing supported by the National Technical Library in Prague. This work was supported by Grant CEITEC VUT-K-22-7769, which is realized within the project Quality Internal Grants of BUT (KInG BUT), Reg. No. CZ.02.2.69/0.0/0.0/19_073/0016948, which is financed from the OP RDE. The results of this research were obtained within the EPSILON project no. TH04020540 supported by the Technology Agency of the Czech Republic.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by MS, KK, MD and TS. Data collection and analysis were performed by MS, KK, ZF, DI, MC, PS and LV. The first draft of the manuscript was written by MS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics approval – the Ethics approval is written in the article – All in vivo testing was done at Veterinary and Pharmaceutical University (currently Faculty of Pharmacy at Masaryk University, Brno) in accordance with the guidelines of SUKL (State Institute for Drug Control) and the Ministry of Health of the Czech Republic. The Scientific Committee for the Protection of Animals at the university approved the in vivo testing. After approval, a partial nephrectomy model in rats was carried out.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Missed acknowledgment section included.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sedlář, M., Kacvinská, K., Fohlerová, Z. et al. A synergistic effect of fibrous carboxymethyl cellulose with equine collagen improved the hemostatic properties of freeze-dried wound dressings. Cellulose 30, 11113–11131 (2023). https://doi.org/10.1007/s10570-023-05499-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05499-9