Abstract
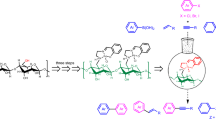
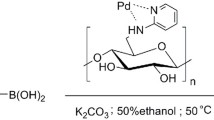
The field of catalysis is ever flourishing to meet the challenges faced in our day-to-day needs keeping in mind the environmental concerns. In line with this quest, a new N-heterocyclic carbene-palladium(II) complex grafted on cellulose, a naturally available biomacromolecule from agricultural waste sugarcane bagasse (Cellu@NHC-Pd) was synthesized as a heterogeneous catalyst. The facile multistep synthesis was achieved using low-cost chemicals and mild reaction conditions. The characterization of the Cellu@NHC-Pd heterogeneous catalyst by various analytical techniques such as FT-IR, FE-SEM, EDS, HR-TEM, TG/DTA, ICP-OES and p-XRD confirmed its structure, morphology, thermal stability and chemical composition. The Cellu@NHC-Pd heterogeneous catalyst was successfully investigated for its catalytic ability in Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions under green reaction medium at ambient temperature. The heterogeneous catalyst was examined for its catalytic effectiveness in the cross-coupling reactions for various parameters like solvent, base, temperature, time and catalyst loading. Additionally, the developed heterogeneous catalyst showed very good tolerance to a variety of functional groups. Being heterogeneous, the catalyst could be easily recovered by simple filtration. The Cellu@NHC-Pd heterogeneous catalyst can be additionally probed for its catalytic excellence in other applications.








Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Antony AM, Kandathil V, Kempasiddaiah M, Sasidhar B, Patil SA, Patil SA (2021) Hexagonal boron nitride supported N-heterocyclic carbene-palladium(II): a new, efficient and recyclable heterogeneous catalyst for Suzuki-Miyaura cross-coupling reaction. Catal Lett 151:1293–1308. https://doi.org/10.1007/s10562-020-03401-x
Antony AM, Kandathil V, Kempasiddaiah M, Dateer RB, Patil SA (2023) Magnetic nanoparticles embedded hexagonal boron nitride tethered N-heterocyclic carbene-palladium(II): an efficient and reusable magnetic catalyst for fluoride-free Hiyama cross-coupling and 4-nitrophenol reduction reactions. J Phys Chem Solids 177:111283. https://doi.org/10.1016/j.jpcs.2023.111283
Balinge KR, Bhagat PR (2019) A polymer-supported salen-palladium complex as a heterogeneous catalyst for the Mizoroki-Heck cross-coupling reaction. Inorganica Chim Acta 495:119017. https://doi.org/10.1016/j.ica.2019.119017
Baran T (2016) Microwave assisted synthesis of biarlys by C-C coupling reactions with a new chitosan supported Pd(II) catalyst. J Mol Struct 1122:111–116. https://doi.org/10.1016/j.molstruc.2016.05.091
Baran T, Nasrollahzadeh M (2020) Cyanation of aryl halides and Suzuki-Miyaura coupling reaction using palladium nanoparticles anchored on developed biodegradable microbeads. Int J Biol Macromol 148:565–573. https://doi.org/10.1016/j.ijbiomac.2020.01.157
Baran T, Baran NY, Menteş A (2018) An easily recoverable and highly reproducible agar-supported palladium catalyst for Suzuki-Miyaura coupling reactions and reduction of o-nitroaniline. Int J Biol Macromol 115:249–256. https://doi.org/10.1016/j.ijbiomac.2018.04.057
Baran NY, Baran T, Nasrollahzadeh M, Varma RS (2019) Pd nanoparticles stabilized on the Schiff base-modified boehmite: catalytic role in Suzuki coupling reaction and reduction of nitroarenes. J Organomet Chem 900:120916. https://doi.org/10.1016/j.jorganchem.2019.120916
Baruah D, Das RN, Hazarika S, Konwar D (2015) Biogenic synthesis of cellulose supported Pd(0) nanoparticles using hearth wood extract of Artocarpus lakoocha Roxb-A green, efficient and versatile catalyst for Suzuki and Heck coupling in water under microwave heating. Catal Commun 72:73–80. https://doi.org/10.1016/j.catcom.2015.09.011
Crudden CM, Allen DP (2004) Stability and reactivity of N-heterocyclic carbene complexes. Coord Chem Rev 248:2247–2273. https://doi.org/10.1016/j.ccr.2004.05.013
D’Alterio MC, Casals-Cruañas È, Tzouras NV, Talarico G, Nolan SP, Poater A (2021) Mechanistic Aspects of the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Chem Eur J 27:13481–13493. https://doi.org/10.1002/chem.202101880
Doke DS, Advani JH, Naikwadi DR, Gawande MB, Walke P, Umbarkar SB, Biradar A (2019) Utilization of waste biomass for the synthesis of functionalizable support for covalent anchoring of active organo catalyst. ACS Sustain Chem Eng 7:3018–3026. https://doi.org/10.1021/acssuschemeng.8b04430
Dong Y, Bi J, Zhang S, Zhu D, Meng D, Ming S, Qin K, Liu Q, Guo L, Li T (2020) Palladium supported on N-Heterocyclic carbene functionalized hydroxyethyl cellulose as a novel and efficient catalyst for the Suzuki reaction in aqueous media. Appl Surf Sci 531:147392. https://doi.org/10.1016/j.apsusc.2020.147392
Fortman GC, Nolan SP (2011) N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem Soc Rev 40:5151–5169. https://doi.org/10.1039/C1CS15088J
Ghiaci M, Zarghani M, Khojastehnezhad A, Moeinpour F (2014) Preparation, characterization and first application of silica supported palladium-N-heterocyclic carbene as a heterogeneous catalyst for C-C coupling reactions. RSC Adv 4:15496–15501. https://doi.org/10.1039/C4RA00002A
Gu X, Qi W, Xu X, Sun Z, Zhang L, Liu W, Pan X, Su D (2014) Covalently functionalized carbon nanotube supported Pd nanoparticles for catalytic reduction of 4-nitrophenol. Nanoscale 6:6609–6616. https://doi.org/10.1039/C4NR00826J
Gujral SS, Khatri S, Riyal P, Gahlot V (2012) Suzuki cross coupling reaction-a review. Indo Glob J Pharm Sci 2:351–367
Heck RF, Nolley J Jr (1972) Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J Org Chem 37:2320–2322. https://doi.org/10.1021/jo00979a024
Hooshmand SE, Heidari B, Sedghi R, Varma RS (2019) Recent advances in the Suzuki-Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem 21:381–405. https://doi.org/10.1039/C8GC02860E
Jin T, Hicks M, Kurdyla D, Hrapovic S, Lam E, Moores A (2020) Palladium nanoparticles supported on chitin-based nanomaterials as heterogeneous catalysts for the Heck coupling reaction. Beilstein J Org Chem 16:2477–2483. https://doi.org/10.3762/bjoc.16.201
Kale D, Rashinkar G, Kumbhar A, Salunkhe R (2017) Facile Suzuki-Miyaura cross coupling using ferrocene tethered N-heterocyclic carbene-Pd complex anchored on cellulose. React Funct Polym 116:9–16. https://doi.org/10.1016/j.reactfunctpolym.2017.04.010
Kandathil V, Fahlman BD, Sasidhar B, Patil SA, Patil SA (2017) A convenient, efficient and reusable N-heterocyclic carbene-palladium(II) based catalyst supported on magnetite for Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions. New J Chem 41:9531–9545. https://doi.org/10.1039/C7NJ01876B
Kandathil V, Koley TS, Manjunatha K, Dateer RB, Keri RS, Sasidhar B, Patil SA, Patil SA (2018) A new magnetically recyclable heterogeneous palladium(II) as a green catalyst for Suzuki-Miyaura cross-coupling and reduction of nitroarenes in aqueous medium at room temperature. Inorganica Chim Acta 478:195–210. https://doi.org/10.1016/j.ica.2018.04.015
Kandathil V, Kempasiddaiah M, Sasidhar B, Patil SA (2019) From agriculture residue to catalyst support; a green and sustainable cellulose-based dip catalyst for C-C coupling and direct arylation. Carbohydr Polym 223:115060. https://doi.org/10.1016/j.carbpol.2019.115060
Kempasiddaiah M, Kandathil V, Dateer RB, Baidya M, Patil SA, Patil SA (2021) Efficient and recyclable palladium enriched magnetic nanocatalyst for reduction of toxic environmental pollutants. J Environ Sci (china) 101:189–204. https://doi.org/10.1016/j.jes.2020.08.015
Khazaei A, Rahmati S, Hekmatian Z, Saeednia S (2013) A green approach for the synthesis of palladium nanoparticles supported on pectin: application as a catalyst for solvent-free Mizoroki-Heck reaction. J Mol Catal A Chem 372:160–166. https://doi.org/10.1016/j.molcata.2013.02.023
Köhler K, Kleist W, Pröckl SS (2007) Genesis of coordinatively unsaturated palladium complexes dissolved from solid precursors during heck coupling reactions and their role as catalytically active species. Inorg Chem 46:1876–1883. https://doi.org/10.1021/ic061907m
Kumar A, Rao GK, Singh AK (2012) Organochalcogen ligands and their palladium(II) complexes: synthesis to catalytic activity for Heck coupling. RSC Adv 2:12552–12574. https://doi.org/10.1039/C2RA20508D
Li Y, Xu L, Xu B, Mao Z, Xu H, Zhong Y, Zhang L, Wang B, Sui X (2017) Cellulose sponge supported palladium nanoparticles as recyclable cross-coupling catalysts. ACS Appl Mater Interfaces 9:17155–17162. https://doi.org/10.1021/acsami.7b03600
Li X, Zhou Z, Wang Y, Dong J, Jia X, Hu Z, Wei Q, Zhang W, Jiang Y, Zhang J (2023) Schiff base modified starch: a promising biosupport for palladium in Suzuki cross-coupling reactions. Int J Biol Macromol 233:123596. https://doi.org/10.1016/j.ijbiomac.2023.123596
Mandal A, Chakrabarty D (2011) Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr Polym 86:1291–1299. https://doi.org/10.1016/j.carbpol.2011.06.030
Mirza-Aghayan M, Mohammadi M, Addad A, Boukherroub R (2020b) Pd nanoparticles supported on reduced graphene oxide as an effective and reusable heterogeneous catalyst for the Mizoroki-Heck coupling reaction. Appl Organomet Chem 34:e5524. https://doi.org/10.1002/aoc.5524
Nahra F, Cazin CS (2021) Sustainability in Ru-and Pd-based catalytic systems using N-heterocyclic carbenes as ligands. Chem Soc Rev 50:3094–3142. https://doi.org/10.1039/C8CS00836A
Niknam E, Panahi F, Khalafi-Nezhad A (2021) Immobilized Pd on a NHC-functionalized metal-organic frameworkmil-101 (Cr): an efficient heterogeneous catalyst in the heck and copper-free Sonogashira coupling reactions. J Organomet Chem 935:121676. https://doi.org/10.1016/j.jorganchem.2020.121676
Peh G-R, Kantchev EAB, Zhang C, Ying JY (2009) N-heterocycle carbene (NHC)-ligated cyclopalladated N, N-dimethylbenzylamine: a highly active, practical and versatile catalyst for the Heck-Mizoroki reaction. Org Biomol Chem 7:2110–2119. https://doi.org/10.1039/B821892G
Polshettiwar V, Len C, Fihri A (2009) Silica-supported palladium: sustainable catalysts for cross-coupling reactions. Coord Chem Rev 253:2599–2626. https://doi.org/10.1016/j.ccr.2009.06.001
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset J-M (2011) Magnetically recoverable nanocatalysts. Chem Rev 111:3036–3075. https://doi.org/10.1021/cr100230z
Rafieian F, Mousavi M, Yu Q, Jonoobi M (2019) Amine functionalization of microcrystalline cellulose assisted by (3-chloropropyl) triethoxysilane. Int J Biol Macromol 130:280–287. https://doi.org/10.1016/j.ijbiomac.2019.01.108
Rahmatpour A (2011) Cellulose sulfuric acid as a biodegradable and recoverable solid acid catalyst for one pot synthesis of substituted pyrroles under solvent-free conditions at room temperature. React Funct Polym 71:80–83. https://doi.org/10.1016/j.reactfunctpolym.2010.11.001
Rajabi F, Schaffner D, Follmann S, Wilhelm C, Ernst S, Thiel WR (2015) Electrostatic grafting of a palladium N-heterocyclic carbene catalyst on a periodic mesoporous organosilica and its application in the Suzuki-Miyaura reaction. ChemCatChem 7:3513–3518. https://doi.org/10.1002/cctc.201500417
Ranganath KV, Onitsuka S, Kumar AK, Inanaga J (2013) Recent progress of N-heterocyclic carbenes in heterogeneous catalysis. Catal Sci Technol 3:2161–2181. https://doi.org/10.1039/C3CY00118K
Sampatkumar HG, Antony AM, Trivedi M, Sharma M, Ghate M, Baidya M, Dateer RB, Patil SA (2022) In situ biosynthesis of palladium nanoparticles on banana leaves extract-coated graphitic carbon nitride: an efficient and reusable heterogeneous catalyst for organic transformations and antimicrobial agent. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03222-5
Selander N, Szabó KJ (2011) Catalysis by palladium pincer complexes. Chem Rev 111:2048–2076. https://doi.org/10.1021/cr1002112
Singh SB, Tandon PK (2014) Catalysis: a brief review on nano-catalyst. J Energy Chem 2:106–115
Thiangtham S, Runt J, Saito N, Manuspiya H (2020) Fabrication of biocomposite membrane with microcrystalline cellulose (MCC) extracted from sugarcane bagasse by phase inversion method. Cellulose 27:1367–1384. https://doi.org/10.1007/s10570-019-02866-3
Vishal K, Fahlman BD, Sasidhar B, Patil SA, Patil SA (2017) Magnetic nanoparticle-supported N-heterocyclic carbene-palladium(II): a convenient, efficient and recyclable catalyst for Suzuki-Miyaura cross-coupling reactions. Catal Lett 147:900–918. https://doi.org/10.1007/s10562-017-1987-7
Wang X, Hu P, Xue F, Wei Y (2014) Cellulose-supported N-heterocyclic carbene-palladium catalyst: synthesis and its applications in the Suzuki cross-coupling reaction. Carbohydr Polym 114:476–483. https://doi.org/10.1016/j.carbpol.2014.08.030
Wang W-J, Roberts F, Peterson S, Ha S, Scudiero L, Coustel R, Mallet M, Abdelmoula M, Ruby C (2022) Iron-iron oxide supported palladium catalyst for the interconversion of formate and carbon dioxide. J Chem Eng 427:131763. https://doi.org/10.1016/j.cej.2021.131763
Xie J, Hse C-Y, Cornelis F, Hu T, Qi J, Shupe TF (2016) Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr Polym 151:725–734. https://doi.org/10.1016/j.carbpol.2016.06.011
Zhou J, Sun H, Xu C, Wang Z, Zhang H, Guo D, Zhang J, Ji X, Liu L, Ma J, Tong Z (2022) Palladium nanoparticles supported on α-zirconium phosphate nanosheets as a highly efficient heterogeneous catalyst for the Heck reaction. J Taiwan Inst Chem Eng 138:104478. https://doi.org/10.1016/j.jtice.2022.104478
Acknowledgments
The authors thank DST-SERB, India (SERB/F/1423/2017–18 (No. YSS/2015/000010), DST-Nanomission, India (No. SR/NM/NS-20/2014) and Jain (Deemed-to-be University), India, for financial support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
All authors have revised the last version of the submitted manuscript and we approve its submission.
Ethics approval
Authors declare that the manuscript is not submitted to any other journal at the time of submission for simultaneous consideration, that the submitted work is original and has not been published elsewhere in any form, that this work is not part of a single study, that results are presented under the principles of honesty, without fabrication falsification or inappropriate data manipulation and that no data, text or theories by others are presented as our own.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Antony, A.M., Chamanmalik, M.I., Kandathil, V. et al. Biomacromolecule supported N-heterocyclic carbene-palladium(II) as a novel catalyst for Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions. Cellulose 30, 7551–7573 (2023). https://doi.org/10.1007/s10570-023-05323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05323-4