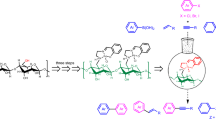
Abstract
Due to their eco-friendly nature, polysaccharides are desirable supporting materials in organic transformations. Nevertheless, as is the case for other supports, polysaccharides have to face the issue of seeking more binding sites via multifunctional structures to capture metal species in the catalyst, which enhance stability and promote catalytic performance in aforementioned process. In this paper, an environmentally-friendly and multifunctional cellulose supported Pd(II)-Schiff base complex is fabricated and applied in the formation of different biaryls under mild ambient conditions. The results of thermal analysis reveal that the composite has high thermal stability. The as-prepared catalyst demonstrates to be a robust and efficient catalyst with more than 90% yields in H2O: EtOH (1:1) at 70 °C by using 0.30 mol% of catalyst under air towards the coupling of various substituted aryl halides and phenylboronic acids. Moreover, identified by ICP-OES analysis, the green Pd(II) catalyst displays higher metal content (1.93%) in comparison with the direct deposition of Pd particles on cellulose (0.93%), and prevents the metal leaching (< 1%) via the coordination interaction of multiple capturing sites (–OH, Schiff base and pyridyl moieties) with palladium. The resultant catalyst is characterized by FT-IR, TGA, XRD, SEM, TEM, XPS, CP/MAS 13C-NMR, and ICP-OES examination. Also, this green catalyst is able to be retrieved in five cycles with simple centrifugation. Notably, we propose a plausible multifunctional catalyst complex. The present study offers a novel and effective supported catalyst, which broadens the contributions of polysaccharides in green catalysis.
Graphic abstract








Similar content being viewed by others
Abbreviations
- Cell:
-
Cellulose
- Cell-NH2 :
-
Silylation cellulose
- Cell-Sb:
-
Schiff base functionalized cellulose
- Cell-Sb-Pd(II):
-
Palladium supported on Schiff base functionalized cellulose
References
Baran T (2017a) Practical, economical, and eco-friendly starch-supported palladium catalyst for Suzuki coupling reactions. J Colloid Interface Sci 496:446–455
Baran T (2017b) A new chitosan Schiff base supported Pd(II) complex for microwave-assisted synthesis of biaryls compounds. J Mol Struct 1141:535–541
Baran T (2018a) Pd(0) nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki–Miyaura cross coupling reaction and reduction of o-nitroaniline. Carbohydr Polym 195:45–52
Baran T (2018b) Ultrasound-accelerated synthesis of biphenyl compounds using novel Pd(0) nanoparticles immobilized on bio-composite. Ultrason Sonochem 45:231–237
Baran T (2019) Highly recoverable, reusable, cost-effective, and Schiff base functionalized pectin supported Pd(II) catalyst for microwave-accelerated Suzuki cross-coupling reactions. Int J Biol Macromol 127:232–239
Baran T, Menteş A (2016a) Highly efficient Suzuki cross-coupling reaction of biomaterial supported catalyst derived from glyoxal and chitosan. J Organomet Chem 803:30–38
Baran T, Menteş A (2016b) Microwave assisted synthesis of biarlys by C–C coupling reactions with a new chitosan supported Pd(II) catalyst. J Mol Struct 1122:111–116
Baran T, Menteş A (2016c) Cationic palladium(II) catalysts on Ocarboxymethyl chitosan Schiff base for Suzuki coupling reactions. J Macromol Sci A 53:687–690
Baran T, Menteş A (2016d) Polymeric material prepared from Schiff base based on O-carboxymethyl chitosan and its Cu(II) and Pd(II) complexes. J Mol Struct 1115:220–227
Baran T, Sargin I, Kaya M, Menteş A (2016) Green heterogeneous Pd(II) catalyst produced from chitosan–cellulose micro beads for green synthesis of biaryls. Carbohydr Polym 152:181–188
Baran NY, Baran T, Menteş A (2017a) Fabrication and application of cellulose Schiff base supported Pd(II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl Catal A Gen 531:36–44
Baran T, Baran NY, Menteş A (2017b) A new air and moisture stable robust bio-polymer based palladium catalyst for highly efficient synthesis of biaryl compounds. Appl Organomet Chem 32:e4076
Baran T, Sargin I, Kaya M, Menteş A (2017c) Design and application of sporopollenin microcapsule supported palladium catalyst: Remarkably high turnover frequency and reusability in catalysis of biaryls. J Colloid Interface Sci 486:194–203
Baran NY, Baran T, Menteş A (2018a) Production of novel palladium nanocatalyst stabilized with sustainable chitosan/cellulose composite and its catalytic performance in Suzuki–Miyaura coupling reactions. Carbohydr Polym 181:596–604
Baran T, Baran NY, Menteş A (2018b) An easily recoverable and highly reproducible agar-supported palladium catalyst for Suzuki–Miyaura coupling reactions and reduction of o-nitroaniline. Int J Biol Macromol 115:249–256
Baran T, Sargin I, Kaya M, Mulerčikasc P, Kazlauskaitė S, Menteş A (2018c) Production of magnetically recoverable, thermally stable, bio-based catalyst: remarkable turnover frequency and reusability in Suzuki coupling reaction. Chem Eng J 331:102–113
Bi J, Chen J, Dong Y, Guo W, Zhu D, Li T (2018) Polymer-supported palladium(II) containing N2O2: an efficient and robust heterogeneous catalyst for C–C coupling reactions. J Polym Sci Part A Polym Chem 56:2344–2353
Cai Z, Kim J (2010) Bacterial cellulose/poly(ethylene glycol) composite:characterization and first evaluation of biocompatibility. Cellulose 17:83–91
Chen W, Zhong L, Peng X, Lin J, Sun R (2014) Xylan-type hemicelluloses supported terpyridine-palladium(II) complex as an efficient and recyclable catalyst for Suzuki–Miyaura reaction. Cellulose 21:125–137
Chen J, Zhang J, Sun W, Song K, Zhu D, Li T (2018) Pd immobilized on polyamide based on melamine and terephalic acid as an efficient and recyclable catalyst for Suzuki–Miyaura coupling reaction. Appl Organomet Chem 32:e4135
Chen X, Qiu X, Hou M, Wu X, Dong Y, Ma Y, Yang L, Wei Y (2019) Differences in zwitterionic sulfobetaine and carboxybetaine dextran-based hydrogels. Langmuir 35:1475–1482
Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coordin Chem Rev 311:1–23
de Leon AC, Chen QY, Palaganas NB, Palaganas JO, Manapat J, Advincula RC (2016) High performance polymer nanocomposites for additive manufacturing applications. React Funct Polym 103:141–155
Dong Y, Wu X, Chen X, Wei Y (2017) N-Methylimidazole functionalized carboxymethycellulose-supported Pd catalyst and its applications in Suzuki cross-coupling reaction. Carbohydr Polym 160:106–114
Du Q, Li Y (2011) Air-stable, recyclable, and time-efficient diphenylphosphinite cellulose-supported palladium nanoparticles as a catalyst for Suzuki–Miyaura reactions. Beilstein J Org Chem 7:378–385
Fang M, Fan G, Li F (2014) Highly efficient palladium nanoparticles homogeneously immobilized on microporous ZnAl2O4 support for Suzuki–Miyaura coupling reaction. Catal Lett 144:142–150
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
Gao W, Razavi R, Fakhri A (2018) Preparation and development of FeS2 Quantum Dots on SiO2 nanostructures immobilized in biopolymers and synthetic polymers as nanoparticles and nanofibers catalyst for antibiotic degradation. Int J Biol Macromol 114:357–362
Godoy F, Segarra C, Poyatos M, Peris E (2011) Palladium catalysts with sulfonate-functionalized-NHC ligands for Suzuki–Miyaura cross-coupling reactions in water. Organometallics 30:684–688
Gupta VK, Fakhri A, Agarwal S, Sadeghi N (2017) Synthesis of MnO2/cellulose fiber nanocomposites for rapid adsorption of insecticide compound and optimization by response surface methodology. Int J Biol Macromol 102:840–846
Hosseini M, Pourabadeh A, Fakhri A, Hallajzadeh J, Tahami S (2018a) Synthesis and characterization of Sb2S3–CeO2/chitosan-starch as a heterojunction catalyst for photo-degradation of toxic herbicide compound: optical, photo-reusable, antibacterial and antifungal performances. Int J Biol Macromol 118:2108–2112
Hosseini M, Sarafbidabad M, Fakhri A, NoorMohammadi Z, Tahami S (2018b) Preparation and characterization of MnS2/chitosan sodium alginate and calcium alginate nanocomposites for degradation of analgesic drug: photocorrosion, mechanical, antimicrobial and antioxidant properties studies. Int J Biol Macromol 118:1494–1500
Jadhav S, Jagdale A, Kamble S, Kumbhar A, Salunkhe R (2016) Palladium nanoparticles supported on a titanium dioxide cellulose composite (PdNPs@TiO2–Cell) for ligand-free carbon–carbon cross coupling reactions. RSC Adv 6:3406–3420
Jamwal N, Sodhi RK, Gupta P, Paul S (2011) Nano Pd(0) supported on cellulose: a highly efficient and recyclable heterogeneous catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols under liquid phase catalysis. Int J Biol Macromol 49:930–935
Jebali Z, Granados A, Nabili A, Boufi S, do Rego AMB, Majdoub H, Vallribera A (2018) Cationic cellulose nanofibrils as a green support of palladium nanoparticles: catalyst evaluation in Suzuki reactions. Cellulose 25:6963–6975
Jing L, Sun J, Sun F, Chen P, Zhu G (2018) Porous aromatic framework with mesopores as a platform for a super-efficient heterogeneous Pd based organometallic catalysis. Chem Sci 9:3523–3530
Kale D, Rashinkar G, Kumbhar A, Salunkhe R (2017) Facile Suzuki–Miyaura cross coupling using ferrocene tethered N-heterocyclic carbene-Pd complex anchored on cellulose. React Funct Polym 116:9–16
Keshipour S, Ahmadi F, Seyyedi B (2017) Chitosan-modified Pd(II)-d-penicillamine: preparation, characterization, and catalyst application. Cellulose 24:1455–1462
Klemm D, Heublein B, Fink H, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Lempers HEB, Sheldon RA (1998) The stability of chromium in CrAPO-5, CrAPO-11, and CrS-1 during liquid phase oxidations. J Catal 175:62–69
Li B, Guan Z, Wang W, Yang X, Hu J, Tan B, Li T (2012a) Highly dispersed Pd catalyst locked in knitting aryl network polymers for Suzuki–Miyaura coupling reactions of aryl chlorides in aqueous media. Adv Mater 24:3390–3395
Li P, Wang L, Zhang L, Wang G (2012b) Magnetic nanoparticles-supported palladium: a highly efficient and reusable catalyst for the Suzuki, Sonogashira, and Heck reactions. Adv Synth Catal 354:1307–1318
Li D, Zhang J, Cai C (2018) Pd nanoparticles supported on cellulose as a catalyst for vanillin conversion in aqueous media. J Org Chem 83:7534–7538
Lin X, Li X, Li F, Fang Y, Tian M, An X, Fu Y, Jin J, Ma J (2016) Precious-metal-free Co–Fe–Ox coupled nitrogen-enriched porous carbon nanosheets derived from Schiff-base porous polymers as superior electrocatalysts for the oxygen evolution reaction. J Mater Chem A 4:6505–6512
Lin B, Liu X, Zhang Z, Chen Y, Liao X, Li Y (2017) Pd(0)–CMC@Ce(OH)4 organic/inorganic hybrid as highly active catalyst for the Suzuki–Miyaura reaction. J Colloid Interface Sci 497:134–143
Littke AF, Dai C, Fu FC (2000) Versatile catalysts for the Suzuki cross-coupling of arylboronic acids with aryl and vinyl halides and triflates under mild conditions. J Am Chem Soc 122:4020–4028
Liu J, He F, Durham E, Zhao D, Roberts CB (2008) Polysugar-stabilized Pd nanoparticles exhibiting high catalytic activities for hydrodechlorination of environmentally deleterious trichloroethylene. Langmuir 24:328–336
Lu ZH, Jasinski JB, Handa S, Hammond GB (2018) Recyclable cellulose–palladium nanoparticles for clean cross-coupling chemistry. Org Biomol Chem 16:2748–2752
Ma R, Yang P, Bian F (2018) Magnetic dendritic polymer nanocomposites as supports for palladium: a highly efficient and reusable catalyst for Mizoroki–Heck and Suzuki–Miyaura coupling reactions. New J Chem 42:4748–4756
Martin R, Buchwald SL (2008) Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc Chem Res 41:1461–1473
Molnár Á, Papp A (2014) The use of polysaccharides and derivatives in palladium-catalyzed coupling reactions. Catal Sci Technol 4:295–310
Mondal B, Mukherjee PS (2018) Cage encapsulated gold nanoparticles as heterogeneous photocatalyst for facile and selective reduction of nitroarenes to azo compounds. J Am Chem Soc 140:12592–12601
Mondal MIH, Sarmina Yeasmin M, Saifur Rahman M (2015) Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int J Biol Macromol 79:144–150
Moon RJ, Martini A, Nairn J, Simonsenf J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Naghipour A, Fakhri A (2016) Heterogeneous Fe3O4@chitosan-Schiff base Pd nanocatalyst: fabrication, characterization and application as highly efficient and magnetically-recoverable catalyst for Suzuki–Miyaura and Heck–Mizoroki C–C coupling reactions. Catal Commun 73:39–45
Nehra P, Khungar B, Pericherla K, Sivasubramanian SC, KumarImidazolium A (2014) Imidazolium ionic liquid-tagged palladium complex: an efficient catalyst for the Heck and Suzuki reactions in aqueous media. Green Chem 16:4266–4271
Ohtaka A, Kawase M, Aihara S, Miyamoto Y, Terada A, Nakamura K, Hamasaka G, Uozumi Y, Shinagawa T, Shimomura O, Nomura R (2018) Poly(tetrafluoroethylene)-stabilized metal nanoparticles: preparation and evaluation of catalytic activity for Suzuki, Heck, and arene hydrogenation in water. ACS Omega 3:10066–10073
Penna A, Careri M, Spencer ND, Rossi A (2015) Effects of tailored surface chemistry on desorption electrospray ionization mass spectrometry: a surface-analytical study by XPS and AFM. J Am Soc Mass Spectrom 26:1311–1319
Pereira PHF, Voorwald HJC, Cioffi MOH, Da Silva MLCP, Rego AMB, Ferraria AM, Pinho MND (2014) Sugarcane bagasse cellulose fibres and their hydrous niobium phosphate composites: synthesis and characterization by XPS, XRD and SEM. Cellulose 21:641–652
Rafiee F, Azam Hosseini S (2018) CNC pincer palladium complex supported on magnetic chitosan as highly efficient and recyclable nanocatalyst in C–C coupling reactions. Appl Organomet Chem 32:e4519
Razavi N, Akhlaghinia B, Jahanshahi R (2017) Aminophosphine palladium(0) complex supported on ZrO2 nanoparticles (ZrO2@AEPH2–PPh2–Pd(0)) as an efficient heterogeneous catalyst for Suzuki–Miyaura and Heck–Mizoroki reactions in green media. Catal Lett 147:360–373
Rezaei G, Naghipour A, Fakhri A (2018) Catalytic performance studies of new Pd and Pt Schiff base complexes covalently immobilized on magnetite nanoparticles as the environmentally friendly and magnetically recoverable nanocatalyst in C–C cross coupling reactions. Catal Lett 148:732–744
Sabaqian S, Nemati F, Heravi MM, Nahzomi HT (2016) Copper(I) iodide supported on modified cellulose-based nano-magnetite composite as a biodegradable catalyst for the synthesis of 1,2,3-triazoles. Appl Organomet Chem 31:e3660
Sabaqian S, Nemati F, Nahzomi HT, Heravi MM (2017) Palladium acetate supported on amidoxime-functionalized magnetic cellulose: synthesis, DFT study and application in Suzuki reaction. Carbohydr Polym 177:165–177
Sabaqian S, Nemati F, Nahzomi HT, Heravi MM (2018) Silver(I) dithiocarbamate on modified magnetic cellulose: synthesis, density functional theory study and application. Carbohydr Polym 184:221–230
Sarkar SM, Lutfor Rahman M, Yusoff MM (2015) Pyridinyl functionalized MCM-48 supported highly active heterogeneous palladium catalyst for cross-coupling reactions. RSC Adv 5:19630–19637
Shen H, Shen C, Chen C, Wang A, Zhang P (2015) Novel glycosyl pyridyl-triazole@palladium nanoparticles: efficient and recoverable catalysts for C–C cross-coupling reactions. Catal Sci Technol 5:2065–2071
Shirase S, Shinohara K, Tsurugi H, Mashima K (2018) Oxidation of alcohols to carbonyl compounds catalyzed by oxo-bridged dinuclear cerium complexes with pentadentate Schiff-base ligands under a dioxygen atmosphere. ACS Catal 8:6939–6947
Tamura M, Yuasa N, Cao J, Nakagawa Y, Tomishige K (2018) Transformation of sugars into chiral polyols over a heterogeneous catalyst. Angew Chem Int Ed 57:8058–8062
Veisi H, Azadbakht R, Saeidifar F, Abdi MR (2017) Schiff base-functionalized multi walled carbon nano tubes to immobilization of palladium nanoparticles as heterogeneous and recyclable nanocatalyst for Suzuki reaction in aqueous media under mild conditions. Catal Lett 147:976–986
Veres M, Koós M, Tóth S, Füle M, Pócsik I, Tóth A, Mohai M, Bertóti I (2005) Characterisation of α-C:H and oxygen-containing Si:C:H films by Raman spectroscopy and XPS. Diam Relat Matee 14:1051–1056
Wang X, Hu P, Xue F, Wei Y (2014) Cellulose-supported N-heterocyclic carbene-palladium catalyst: synthesis and its applications in the Suzuki cross-coupling reaction. Carbohydr Polym 114:476–483
Wang X, Xu Y, Wang F, Wei Y (2015) Functionalized cellulose-supported triphenylphosphine and its application in Suzuki cross-coupling reactions. J Appl Polym Sci 132:41427
Wang C, Salmon L, Ciganda R, Yate L, Moya S, Ruiza J, Astruc D (2017) An efficient parts-per-million α-Fe2O3 nanocluster/graphene oxide catalyst for Suzuki–Miyaura coupling reactions and 4-nitrophenol reduction in aqueous solution. Chem Commun 53:644–646
Widegren JA, Bennett MA, Finke RG (2003) Is it homogeneous or heterogeneous catalysis? Identification of bulk ruthenium metal as the true catalyst in benzene hydrogenations starting with the monometallic precursor, Ru(II)(Ł6-C6Me6)(OAc)2, plus kinetic characterization of the heterogeneous nucleation, then autocatalytic surface-growth mechanism of metal film formation. J Am Chem Soc 125:10301–10310
Wu X, Chen X, Hu P, Hou M, Dong Y, Wei Y (2018) Antifouling zwitterionic dextran micelles for efficient loading DOX. Carbohydr Polym 191:136–141
Xiao J, Lu Z, Li Y (2015) Carboxymethylcellulose-supported palladium nanoparticles generated in situ from palladium(II) carboxymethylcellulose: an efficient and reusable catalyst for Suzuki–Miyaura and Mizoroki–Heck reactions. Ind Eng Chem Res 54:790–797
Xiong G, Chen X, You L, Ren B, Ding F, Dragutan I, Dragutan V, Sun Y (2018) La–metal–organic framework incorporating Fe3O4 nanoparticles, post-synthetically modified with Schiff base and Pd. A highly active, magnetically recoverable, recyclable catalyst for C–C cross-couplings at low Pd loadings. J Catal 361:116–125
Xu S, Song K, Li T, Tan B (2015) Palladium catalyst coordinated in knitting N-heterocyclic carbene porous polymers for efficient Suzuki–Miyaura coupling reactions. J Mater Chem A 3:1272–1278
Yuan D, Chen L, Yuan L, Liao S, Yang M, Zhang Q (2016) Superparamagnetic polymer composite microspheres supported Schiff base palladium complex: an efficient and reusable catalyst for the Suzuki coupling reactions. Chem Eng J 287:241–251
Zhang J, Zhao G, Popovic Z, Lu Y, Liu Y (2010) Pd-porphyrin functionalized ionic liquid-modified mesoporous SBA-15: an efficient and recyclable catalyst for solvent-free Heck reaction. Mate Res Bull 45:1648–1653
Zhang Q, Su H, Luo J, Wei Y (2013) Recyclable palladium(II) imino-pyridine complex immobilized on mesoporous silica as a highly active and recoverable catalyst for Suzuki–Miyaura coupling reactions in aqueous medium. Tetrahedron 69:447–454
Zheng Z, Sun J, Fakhri A, Surendar A, Ibatova AZ, Liu J (2018) Synthesis, photocatalytic, optical, electronic and biological properties of the CoS2–CuS on cellulose nanocomposites as novel nano catalyst by a sonochemical technology. J Mater Sci Mater Electron 29:18531–18539
Acknowledgments
We appreciatively acknowledge the funding support received from the National Natural Science Foundation of China (Grant No. 21473064). We are also grateful to the Analytical and Testing Center of Huazhong University of Science and Technology for allowing us to use the facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, Y., Bi, J., Zhu, D. et al. Functionalized cellulose with multiple binding sites for a palladium complex catalyst: synthesis and catalyst evaluation in Suzuki–Miyaura reactions. Cellulose 26, 7355–7370 (2019). https://doi.org/10.1007/s10570-019-02568-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02568-w