Abstract
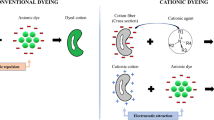
Cationisation of cellulose fibres has been studied extensively as an alternative to permit salt free reactive dyeing, however chemical balances of the cationisation reaction often are not taken into account. The chemical consumption of cationisation processes described in the literature is substantial and often over-compensates savings in salt in a conventional reactive dyeing. Besides very high consumption of chemicals the high level of cationisation generates problems of uneven dyeings and shade variations. In a new approach low concentrations of 3-chloro-2-hydroxypropyl-N,N,N-trimethylammonium chloride (CHPTAC) were applied in a pad batch cationisation process for cotton fabric to achieve a low level cationisation. The cationic group content of the processed fibres ranged from 5 to 79 mmol kg−1. Exhaust dyeing experiments with Reactive Blue 19 demonstrated that a salt free reactive dyeing is possible with low cationic group content 14 mmol kg−1. The optimised low-level cationisation reduces the overall chemical consumption of the reactive dyeing, and thus represents a cleaner alternative to present reactive dyeing operations which are based on addition of high amounts of salt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an annual production of 23×106 metric tons cotton fibres are the most important representative for cellulose based textile fibres. In almost 60% of the cases reactive dyes are used for coloration of these fibres, which then sums up to an annual production of 270,000 metric tons of reactive dyes (Burkinshaw et al. 2019).
During dye fixation a covalent bond between the reactive dyes and the cellulose fibre is formed. The competitive hydrolysis of the anchor group in the alkaline dyebath leads to substantial losses in dye utilisation. Thus in exhaust dyeing dye fixation rates between 60 and 80% of the total added reactive dye are realistic values. High concentrations of salt (NaCl, Na2SO4) up to 50 g L−1 are used to increase dye exhaustion and fixation. As an example, when 50 g L−1 NaCl is used in reactive dyeing of 1 kg of cotton at a mass to liquor ratio of 1:10, an amount of 0.5 kg salt is released with the spent dyebath (Bechtold and Pham 2019). As a result of the limited dye fixation and the high salt concentration highly coloured waste water is released from reactive dyeing, which in addition contains substantial amounts of salt (Turcanu and Bechtold 2017).
Several strategies to improve the dye fixation in reactive dyeing have been proposed in the literature and a few also have been installed in technical scale.
The use of double anchor reactive dyes led to a substantial improvement in dye fixation, however still addition of salt is required and limited dye fixation lead to release of intensively coloured waste water (Haque et al. 2015).
A reduction of the dyebath volume reduces the homogeneous hydrolysis reaction in the dyebath accordingly. This approach has been realised in the so-called airflow® dyeing machine where a mixture of air and liquid dyebath used to transport the goods in a jet-type dyeing machine (Abate and Tadesse 2021). Considerable abrasive load for the goods and limited dyestuff solubility in the low dyebath volume restricted a wider use of this machine. Also addition of silicone oil or plant oil based liquids to the dyebath has been proposed to reduce the total volume of aqueous dyebaths (Seemork et al. 2020, 2021). The removal of these additives both from the dyed goods and from the spent dyebath require additional processing to prevent release of the oily phases into the waste water (Haque et al. 2015).
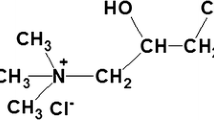
Another approach to increase dye fixation utilises the chemical modification of the cellulose fibre by cationisation (Zhang et al. 2021; Li et al. 2022). A wide range of chemicals have been studied for the cationisation of cotton fibres through incorporation of quaternary ammonium groups (Pruś et al. 2022; Correia et al. 2020). As representative examples N-oxiranylmethyl-N-methylmorpholinium chloride and 2-oxiranylpyridine have been investigated as etherification agents to introduce cationic groups in cellulose (Hsani et al. 2009). The most important representative for cationic modification of cotton is 3-chloro-2-hydroxypropyl-N,N,N-trimethylammonium chloride (CHPTAC), which also is used in technical scale operations. The degree of cationisation then is assessed through dye sorption experiments, however quantification of the cationic sites e.g. through N-analysis is performed rarely. Representative levels of N-content in cationised fibres are between 0.1 and 0.3%wt nitrogen (Correia et al. 2020).
For continuous treatment combination of cationisation with plasma pretreatment to replace bleach and scouring processes and combination of cationisation with pad-steam dyeing have been investigated (Correia et al. 2021; Wang et al. 2022). Two major problems of the current methodology however lower their potential for wider use to achieve sustainable and low polluting reactive dyeing:
-
The presence of quaternary ammonium groups in the cellulose fibre leads to rapid dye sorption most probably via an ion-exchange mechanism followed by the dye fixation close to the site of initial cationic binding (Pruś et al. 2022). The high amount of cationic groups leads to very rapid dye sorption, strong binding of the dye and complete bath exhaustion. As an example almost complete dye exhaustion was observed on cationised microcrystalline cellulose with in less than 5 min (Hashem and El-Shishtawy 2001). As a consequence of the strong binding forces the tendency for levelling out uneven distributed dye is low, in particular when competitive sorption in dye mixtures occurs (Farrell et al. 2014). Such dyeing processes thus are difficult to operate and dyeing of light to medium shades is very challenging or impossible.
-
In addition the chemical efficiency of the preceding cationisation processes is low and only a minor share of the total amount of cationisation agent is utilised through fixation on the cellulose (Nallathambi and Venkateshwarapuram Rengaswami 2016). An unacceptably high amount of chemicals is spent during the fibre cationisation, which lowers the ecological profile of the overall dyeing process substantially (Correia et al. 2020; Kanik and Hauser 2002). Representative values for the specific consumption of CHPTAC are at 100 g technical product per 1 kg of cotton fibres in pad-batch techniques, for exhaust processes even higher amounts e.g. 30%wt of CHPTAC related to the mass of fibre are spent (Hashem 2006; Correia et al. 2021; Zhang et al. 2018).
In this work we follow the hypothesis that use of a very low level of cationisation could support the initial sorption of the reactive dye to an extent which will allow reduction of the salt load used in conventional dyeing. The compensation of the negative charges from dissociated carboxylic groups by cationic groups should reduce the charge repulsion between the anionic reactive dye and the cellulose surface. As a result sorption of the reactive dye on the cellulose should increase. The sorption of the dyes however still should permit sufficient dye mobility which is a condition for dye levelling and uniform dyeing. Low level cationisation of cotton could reduce the chemical consumption reported in the literature substantially and thus make reactive dyeing of cationised cellulose fibres become a real ecological alternative to a conventional reactive dyeing with high salt consumption or cationisation processes described in the literature.
Cotton fibres were cationised with 3-chloro-2-hydrodxypropyl-N,N,N-trimethylammonium chloride in a pad-batch process. Fibres with different levels of cationisation were prepared and characterised by ζ-potential measurement, FTIR and N-content. Sorption experiments with C.I. Reactive Blue 19 were performed without salt to analyse the sorption behaviour as function of cationisation. A mono-functional reactive dye with chemically resistant anthraquinoid chromophore was chosen as model. Technically the bi-functional reactive dye C.I. Reactive Black 5 would be more relevant however the two anchor groups and the rather sensitive double-azo-chromophore would make the investigations and model formulation more complex. Dye extraction was used to study the binding of the dye on the fibres and to distinguish between dye sorption and dye fixation. Salt free dyeing experiments then were used to demonstrate the effect of the low level cationisation on the process efficiency.
Model calculations to compare the new process to reference processes with use of salt to increase dye fixation are given and discussed.
Experimental
Materials and chemicals
Bleached cotton fabric (plain weave, mass per area 108 g m−2, warp 61.5 ± 0.7 yarns cm−1, weft 35 ± 0.5 yarns cm−1) was used for the cationisation experiments. 3-chloro-2-hydroxypropyl-N,N,N-trimethylammonium chloride, (60%wt aqueous solution, Sigma-Aldrich) was used as cationisation agent. C.I. Reactive Blue 19 (RB19, MW=626.5 g mol−1, Remazol Brillant Blue R, dye content=50%wt, DyStar, Frankfurt, Germany), a mono-functional vinylsulphone type reactive dye was used (Fig. 1).
All other chemicals used (NaOH, NaCl, KCl, KOH, Na2CO3, DMF, HCl, NaOAc, sodium silicate) were reagent grade chemicals.
Cationisation
In a first series the fabric samples were treated with the cationisation agent without further pretreatment (Table 1, solution 1–4). In a second series (Table 1, solution 5–8) an acid pretreatment (0.5%wt HCl, 0.14 M, liquor ratio (M:L) of 1 g: 35 mL, 1 h, 40 °C) was introduced to remove any acid soluble N-containing substances and thus increase sensitivity of the N-analysis for lower N-content. The samples then were rinsed in deionised water for 1 h and neutralised by means of an aqueous solution of NaOAc (1 g L−1, 12 mM, M:L 1 g: 35 mL, 30 min), followed by a final rinse with deionised water and air drying at ambient temperature.
For cationisation three fabric samples with a total mass of 20.4 g were immersed into 250 mL of the respective solution (Table 1), then excess of liquid was squeezed of by means of a padder to a pick-up of 82%. 1 g L−1 wetting agent (Collasol CDA, CHT, Tuebingen, Germany) was added to each solution. The wet samples were rolled around glass rods, enveloped in plastic film, and turned on a laboratory roller shaker for 24 h. Then the samples were rinsed with soft water and air dried at ambient temperature. The cationisation was performed in three repetitions each recipe given in Table 1.
Nitrogen analysis
To evaluate the degree of cationisation a nitrogen analysis was carried out (Nitrogen Analyser rapidN III, Elementar Analysensysteme GmbH, Langenselbold, Germany). In bleached cotton fabric only presence of bound CHPTAC will lead to substantial nitrogen content. Therefore the reaction efficiency of cationisation and quantification of the cationic groups present in the modified cellulose can be calculated from the nitrogen content of the modified samples. For analysis an accurately weighted mass of 200–250 mg fabric was folded and placed in a tin foil. The tin foil was pressed to the form of a tablet with use of a hand press cell. The tablet then was burned in the nitrogen analyser, which used the Dumas method for nitrogen determination.
Sorption experiments
C.I. Reactive Blue 19 (RB19) was used as representative reactive dye. To avoid any dye fixation through reaction with the cellulose fibres, the pH of the solutions was held at pH 6–8 and no salt was added to the exhaustion baths.
Evaluation dye uptake versus time
A high mass to liquor ratio of M:L 1:400 was used in the experiments to monitor the dye uptake with time. A mass of 0.5 g of cationised fabric was placed on a cylindrical plastic holder in 200 mL 0.05 g L−1 (0.04 mM) RB19 solution to achieve uniform accessibility of RB19 to the whole fabric. The dyebath was pre-heated to 30 °C, then the fabric was immersed into the bath (t = 0 min) and a volume of 0.9 mL dyebath was analysed for the dye content in regular intervals (2, 5, 10, 20, 30, 40, 50, 60, 90, 120, 180, 240 min, 24 h). Changes in the absolute dye content due to sampling were considered in the calculations. The dye concentration in the dyebath was determined by photometry at the wavelength of maximum absorption (695 nm) (Photometer ZEISS MCS 600 equipped with MCS601 UV-NIR C Spectrometer and CLH600 lamp 360–1015 nm). The dye content of the fibres then was calculated from the decrease in dye concentration in the dyebath.
Equilibrium sorption: determination of sorption isotherms
A mass of 0.2 g fabric was treated in 80 mL of dye solution (concentration of 0.01, 0.02, 0.04, 0.08, 0.12, 0.15, 0.2 g L.−1; 0.008, 0.016, 0.032, 0.064, 0.096, 0.120, 0.160 mM) at M:L of 1:400 for 24 h at a temperature of 30 °C. After 24 h the equilibrium concentration of RB19 in the exhausted dye bath was determined by photometry. The obtained isotherms were fitted to the Langmuir and Freundlich isotherms (no weighting of data).
where q (mmol kg−1) is the amount of adsorbed dye after 24 h sorption, Ceq the equilibrium dye concentration in the dyebath (mM), KF the Freundlich coefficient, n the Freundlich, exponent, M (mmol kg−1) the fibre saturation value, KL the Langmuir coefficient (L mmol−1).
Zeta potential measurement
For zeta potential measurements 0.5 g of dry fabric was cut in small pieces and placed in the measuring cell of the zeta potential analyser (SurPASS 3, Anton PaaR GmbH, Graz, Austria). A solution containing 1 mM KCl and 1 mM KOH was used as streaming solution. The measurement thus was started at an alkaline pH and a zeta potential vs. pH curve titration then was carried out with addition of 0.05 M HCl. The step size was adjusted to achieve a pH change of 0.25 to 0.3 units per titration step. For zeta-potential measurements the packing density of the sample is very important, thus always the same packing density was applied. This was controlled with determination of the device intern permeability index.
Dye fixation and extraction
Dyeing-fixation experiments
To assess the performance of a cationised fabric in reactive dyeing a technical reactive dyeing process was simulated. Six samples were selected for this experiment (Table 6). Colour fixation on four different cationised fabrics was compared to dyeings with untreated fabric in presence and without addition of salt (50 g L−1 NaCl). A mass of 1 g fabric was treated with 0.01 g L−1 (0.008 M) RB19 dye solution in deionised water at a M:L of 1:400 (Fig. S1: Temperature–time diagram of the process). The dyebath was pre-heated to 50 °C, for sample 2 the required amount for 50 g L−1 salt was added, and then the fabric was added. After 10 min of pre-soaking 4 mL of RB19 stock solution (10 g L−1, 8 mM) was added. The process then was continued for 60 min to achieve uniform dye sorption. The dye fixation then was initiated by stepwise addition of alkali. At first 30 mL Na2CO3 solution (4 g L−1, 38 mM) was added, after 15 min a volume of 16 mL NaOH solution (69 g L−1, 1.73 M) was added and the process was continued for 1 h to complete dye fixation. The dyed samples then were rinsed at 60 °C with deionised water until washing solutions were colourless. The absorbance of the exhausted dyebath was measured by spectrophotometry and the K/S values of dyed fabric were determined by colour measurement (400–700 nm, specular component excluded, d/8 spectrophotometer equipped with pulsed xenon lamps as light source, Model CM 3610d, Konica Minolta, Japan). The diameter of the measurement area was 8 mm. The reflectance data were transformed to L*, a*, and b* coordinates of the CIE colour space (D65 illuminant, 10° observer) with the onboard software. The colour depth (K/S) was estimated with the Kubelka–Munk function (K/S = [(1 − R)2/2R]), where R is the fractional reflectance at the reflectance maximum (600 nm).
Extraction experiments
Unbound or adsorbed hydrolysed RB19 was extracted from the fibres with a solution containing 50%vol DMF in an aqueous NaCl solution (5 g L−1 NaCl). Extractions were performed with 0.05 g dyed fabric in 10 mL of the extract solution for 30 min at 100 °C. The extraction was repeated until the extraction solution was colourless. In case no dye fixation had occurred the specimen appeared colourless.
ATR-FTIR spectroscopy
For the ATR-FTIR spectroscopy of the fabrics three random spots from the fabric were selected to take some inhomogeneity in cationisation into account. The FTIR-spectra are presented as averaged spectra from the three single measurements (Bruker Invenio FTIR spectrometer equipped with an ATR-unit, Billerica, Massachusetts, U.S.).
Results and discussion
Cationisation
A general reaction scheme of the cationisation reaction is given in Fig. 2a. In Fig. 2b the dye sorption and fixation reaction of RB19 are shown. The formation of an epoxide intermediate generally is accepted as first reaction step in the aqueous alkaline solution (Goclik et al. 2004). The epoxide then reacts in a heterogeneous reaction with the hydroxyl groups of the cellulose and as a result a cationic group is linked covalently to the cellulose. The homogeneous hydrolysis of the epoxide lowers the reaction efficiency of the cationisation reaction as an unwanted side reaction. In the bleached cellulose fibres only very low nitrogen content are present, thus the fibre cationisation can be quantified through analytical determination of the nitrogen content. The cationic sites are formed by quaternary ammonium groups thus the molar content of nitrogen also corresponds to the amount of cationic groups. In Table 1 the composition of cationisation solutions is given and from the liquor pick-up in padding the theoretically added amount of cationisation agent in terms of N-content is given. By comparison of the analytically determined nitrogen content with theoretical maximum reaction efficiency for the cationisation can be calculated. A molar ratio of NaOH: CHPTAC of 2: 1 was chosen for the highest concentration of CHPTAC (expt. 3) (Farrell et al. 2014; Hashem et al. 2003; Correia et al. 2020). The same alkali concentration then was used for experiments 1 and 2 to maintain cellulose fibre swelling at the same level. In the first set of experiments (expt. 1–4) rather high concentrations of chemicals were used, however the reaction efficiency was low with values between 4.5 and 9.2% nitrogen content. For recipe 4 a combination of NaOH and sodium silicate was used, to simulate alkali conditions applied in cold pad batch reactive dyeing and thus increase reaction efficiency of the cationisation. Reaction efficiency however still remained at a low level of 6.4%. As a result of the high concentration of chemicals used in expts. 1–4 the content of cationic groups in the fibre reached values between 10.4 and 79.4 mmol kg−1. This level of cationisation already exceeds the concentration of carboxylic groups in bleached cotton fibres, which is in the dimension of 20 mmol kg−1 (Hirosawa et al. 2001). In the second series of experiments (expt. 5–8) lower concentrations of CHPTAC and NaOH were used. Remarkably the efficiency of the cationisation process increased to values between 20.9 and 29.9%. The molar ratio of NaOH to CHPTAC was comparable for both series of experiments. The increase in reaction efficiency most probably is due to the reduction in alkali concentration, which affects the undesirable hydrolysis of CHPTAC (Eq. 3) to a higher extent than the rate of the covalent binding to the cellulose fibre (Eq. 2) (Hashem et al. 2003). When hydroxyl ions are involved in the hydrolysis reaction of the intermediately formed epoxide a reduction of the alkali concentration will directly reduce the reaction rate of this process. Thus an increase in reaction efficiency up to 29.9% was observed, which is in agreement to literature data presented by (Hashem et al. 2003).
The cationised samples then were analysed by FTIR spectroscopy using an ATR unit. Spectra of untreated cotton and two representative samples with highest degree of cationisation (sample 3 and 8) are shown in Fig. 3.
The FTIR spectrum of the untreated fibres represents a typical absorbance pattern for cellulose. The absorbance between 3500 and 3000 cm−1 is characteristic for the stretching vibration of the O–H groups and the absorbance near 2900 cm−1 results from the C–H stretching vibration. C–H and CH2 bending vibrations lead to absorbances between 1500 and 1100 cm−1, the C–O stretching vibration leads to an absorbance near 1100 cm−1 (Široký et al. 2010). The vibrational pattern of the cationic groups is very similar to the FTIR absorbances of cellulose. The covalently bound 3-trimethyl-ammonium-2-hydroxy-propyl ether will exhibit absorbances for O–H groups, C–H and CH2 groups, and C–O bonds. Thus no visible changes in the FTIR spectra of the cationised fabrics are expected at the rather low content of cationic groups in the modified fibres.
Rate of dye sorption and sorption equilibrium
The rate of dye exhaustion onto the cationised fibre and sorption isotherms was determined to evaluate the effect of the cationisation on the dye sorption. In a first series of experiments the sorption of the RB19 on cationised fibres was studied at a temperature of 30 °C as function of time of immersion. A 0.04 mM dye solution was used at a liquor ratio M:L of 1:400. Thus for 1 kg of material a total amount of 16 mM of dye were available for sorption. In Fig. 4 the time dependent uptake of RB19 into different cationised samples is shown. The time needed to reach a plateau in exhaustion increased with level of cationisation. Under the chosen experimental conditions the untreated fibre did not show any significant dye sorption. The line at dye uptake of 16 mmol kg−1 indicates complete dye exhaustion. Remarkably only sample 3 with a cationic group content of 79 mmol kg−1 reached this limit of full dye exhaustion after 24 h of immersion. For the other cationised fibre samples the dye sorption stagnates at approximately 50% of the theoretical capacity given by the analytically determined cationic group content. As long as RB19 does not enter in dye fixation reaction, the dye bears two negatively charged groups, a sulphonate group and the sulphate group. The results indicate an involvement of both anionic groups present in the dye molecule into the sorption process, thus two cationic sites will be occupied by one dye molecule (Fig. 2b).
A comparison between the content of cationic groups and the dye sorption after 24 h is given in Table 2.
For a more detailed analysis of the dye sorption under equilibrium conditions sorption isotherms were recorded (Fig. 5). The sorption behaviour is characteristic for a Langmuir type sorption. At low concentrations intensive dye sorption occurs, which rapidly stabilises when fibre saturation is reached.
Results of the maximum dye uptake after 24 h of sorption and fitting of the isotherm to a Langmuir isotherm as function of fibre cationisation are given in Table 3.
In Fig. 6a the direct relationship between the saturation value M and the degree of cationisation is observed. Up to a cationisation of 30 mmol kg−1 the slope of the graph is 0.5, which confirms the assumption that un-hydrolysed RB19 occupies two positively charged sites on the fibre. No dye fixation occurred during the sorption experiments. The sorbed dye could be removed completely by extraction with an aqueous DMF solution containing 2.5 g L−1 NaCl. Photographs of samples after dye sorption and also after dye extraction are shown in Fig. 9.
For the application in dyeing processes the increase in dye uptake should also lead to darker colour. The colour depth can be measured by determination measurement of the diffuse reflectance of the dyed samples and the calculation of the Kubelka–Munk value K/S. In Fig. 6b the linear relation between photometrically determined dye uptake and K/S value of the respective sample and the nitrogen content are shown. Therefore in this study K/S values also serve as measure for the dye content of a sample.
Zeta potential measurements
Zeta potential measurements give an indication of the surface charge of a fibre. Unmodified cellulose fibres exhibit negative zeta potential in the pH range between 3 and 10. The dissociation of carboxylic groups increases the negative charge of the fibre surface and thus leads to more negative zeta potential. Functionalisation of the cellulose with cationic groups partially compensates the negative charge of the anionic groups however the increase in zeta potential is not sufficient to obtain positive zeta potential values (Fig. 7). The high dye sorption observed with the cationised samples thus cannot be explained only on the basis of a substantially lowered repulsion of negatively charged dye molecules from the fibre surface.
The cationisation leads to distinct changes in the value for the isoelectric pH (IEP) (Fig. 8a). The correlation between dye uptake and IEP is shown in Fig. 8b. However low differences in zeta potential were measured at pH 6–8 which was applied in the dye sorption experiments. This is an indication that the sorption via ion-exchange mechanism is of higher impact for dye sorption than the change in fibre surface charge.
Dye fixation and dyeing experiments
The aim of these experiments was to investigate if low level cationisation would allow salt free reactive dyeing compared to reference processes with use of 50 g L−1 NaCl (Kunze and Karagiozidis 1999).
The results of the sorption experiments without dye fixation demonstrated the positive correlation between cationisation and dye sorption. A strong binding of the negatively charged dye to the cationic sites however could lead to low dye mobility and thus prevent a successful dye fixation. As a consequence the anchor group would hydrolyse only and the dye would be bound on the fibre via an ion-exchange mechanism. Constant bleeding of the hydrolysed dye during wash operations and low water fastness would be the consequence.
A set of samples thus was dyed according to standard recipe with use of NaCl (Kunze and Karagiozidis 1999) and compared to representative cationised samples (Table 6). For comparison also the colour depth of the respective specimen obtained from the sorption experiments without fixation are given. Only a very light dyeing was obtained in the standard procedure without use of NaCl. With addition of 50 g L−1 NaCl to the dyebath a K/S value of 2.33 is obtained, which reduces after removal of the hydrolysate to K/S 2.18. Cationisation leads to substantially higher bath exhaustion and successful dye fixation. Thus already at a cationic group content of 19 mmol kg−1 a higher K/S value of 2.72 was obtained, which reduces to K/S 2.44 after extractive removal of the hydrolysate.
Due to the high M:L ratio the colour depth of the reference dyeings with salt and the dyeing on 19 mmol kg−1 cationised fabric are both at the level of a 1% dyeing (K/S 2.74) presented in the colour chart of the dye manufacturer (Kunze and Karagiozidis 1999). Higher degree of cationisation to 79 mmol kg increases dye sorption to K/S 9.13 which is even at the level of the 4% dyeing of the colour chart (K/S 9.8) despite the long M:L ratio of 1:400 used. The high content of cationic groups however then leads to the known problems of extremely high sorption rate and uneven dyeing, which prevent successful implementation into robust technical scale processes.
In Fig. 9 photographs of the dyed samples and of samples after extraction of unfixed dye are shown. For the dyed samples the removal of hydrolysed dye does not lead to a substantial loss in colour depth. Specimen from sorption experiments contain only sorbed reactive dyes, which can be removed completely by DMF/water extraction.
Photographs of samples from dyeing experiments A–F, and sorption experiments (5–79 mmol kg−1 N) before (large sample) and after dye extraction (small sample), and representative examples of standard dyeings from the colour chart of the dye manufacturer (bleached cotton tricot, automet process with progressive dosage of alkali, M:L 1:10) (Kunze and Karagiozidis 1999)
Process balances and savings for salt free dyeing of cationised fibres
For a comparative assessment of the new dyeing process a comparison to the state of the art techniques of reactive dyeing is required. The major difference in both processes will arise from their respective consumption of chemicals and water. As already shown in Fig. 9 and Table 4 comparable colour depths were obtained both for a standard reactive dyeing with salt and salt free dyeing of fibres with a cationic group content between 10 and 19 mmol kg−1. Cationisation to a level of 14 mmol kg−1 N was chosen for comparison of chemical consumption and dyeing results to a state of the art reactive dyeing with use of salt. In Table 5 a schematic presentation of both processes and the respective consumption of chemicals and water are presented. A continuous process has been considered for cationisation, followed by an exhaust dyeing process. In addition the chemical consumption of a recently published scientific study for cationisation/dyeing are given for comparison.
The comparison between the standard reactive dyeing (Table 5, process B) and the low level cationisation process investigated in this work (Table 5, process A) clearly demonstrates the importance to limit the chemical consumption during cationisation. Based on the results presented in this study the cationisation of 100 kg cotton fabric will require 0.58 kg CHPTAC, 1.76 kg NaOH and 1080 L water. The same dyestuff and alkali consumption was considered for the dyeing, however addition of 50 kg NaCl is required for the conventional dyeing. The reaction efficiency of the CHPTAC treatment was determined with 21–30%, thus substantial reduction of the CHPTAC consumption can be expected from an optimisation of this step.
The advantages of the low-level cationisation for the overall balance in the chemical consumption also can be demonstrated by comparison to recently published data (Table 5, process C and D). The cationisation of 100 kg cotton fabric therein consumed 13–65 kg CHPTAC, 5.5–27.6 kg NaOH and 4000 L water. The salt free reactive dyeing saves 140–200 kg of Na2SO4 (Zhang et al. 2021), however a considerable amount of chemicals 0.19–0.93 kg of chemicals are spent for cationisation of 1 kg goods. As a consequence of a high level of cationisation, a positive zeta potential is measured. The high number of cationic sites leads to rapid dye sorption and a low tendency of bound dye to migrate and level out concentration differences. Ring dyeings form as the complete dye already occurs in the outer layer of the yarn (Zhang et al. 2021). The rapid dye sorption and the low ability of a cationised fabric to permit dye levelling lead to substantial difficulties in technical scale dyeing.
Besides the positive chemical balances, the application of CHPTAC in a separate treatment step will also contribute significantly to the overall process costs. While savings in dye consumption have been cited as economic advantage, there remains space for future optimisation of the presented concept of low level cationisation by use of other cationisation agents e.g. polycations (Farrell et al. 2014; Correia et al. 2020).
Conclusions
Reaction of CHPTAC with cotton fibres leads to introduction of cationic groups into the carbohydrate structure. Application of low concentrations of CHPTAC in an efficient pad-batch fixation process leads to a low cationic group content in the dimension of 20–30 mmol kg−1. Zeta potential measurement indicated a shift of the isoelectric pH with N-content, however at neutral pH all cationised cellulose fibres still exhibited a negative zeta potential.
Sorption and dye fixation experiments with C.I. Reactive Blue 19 indicated that rapid and high sorption of the dye to the fibre occurs. The sorption isotherms follow a Langmuir isotherm. This equilibrium is a condition to achieve levelling out of uneven dye distribution before dye fixation is initiated by addition of alkali. The fibre saturation value indicates that both negatively charged groups in the RB19 molecule contribute to the sorption process. Therefore one dye molecule occupies two cationic sites on the fibre.
Model dyeings in absence of salt demonstrate that the increased dye concentration at the fibre surface results in high dye fixation rates.
Calculations of chemical balances and water consumption indicate the potential of the technique. The new process requires as chemicals only 0.58 kg CHPTAC and 1.76 kg NaOH for processing of 100 kg cotton fabric, compared to a consumption of 50 kg NaCl in a standard reactive dyeing. In the literature a representative process for cationisation of cotton requires 13–65 kg CHPTAC, 5.5–27.6 kg NaOH for 100 kg fabric (Zhang et al. 2021). This corresponds to a 90% reduction in chemical consumption. Low-level cationisation thus represents a clean approach to reduce the salt load in reactive dyeing, without the negative ecological impact of a high chemical consumption in the preceding cationisation step. Improvement of the cationisation reaction and adaptation of the dyeing process will improve the ecological profile of the process further. Ongoing research addresses process optimisation and scale up of the process. These studies also should address release of cationic substances into the dyebath and possible dye precipitation as well as adverse effects of the treatment on final physical properties of the product e.g. handle and drape.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Abate MT, Tadesse MG (2021) Airflow, foam, and supercritical carbon dioxide dyeing technologies. In: Innovative and emerging technologies for textile dyeing and finishing. Scrivener Publishing LLC, Beverly, USA, pp 137–164. https://doi.org/10.1002/9781119710288.ch5
Bechtold T, Pham T (2019) Textile chemistry. De Gruyter, Berlin
Burkinshaw SM, Liu K, Salihu G (2019) The wash-off of dyeings using interstitial water Part 5: Residual dyebath and wash-off liquor generated during the application of disperse dyes and reactive dyes to polyester/cotton fabric. Dye Pigment 171:106367.
Correia J, Rainert KT, Oliveira FR et al (2020) Cationization of cotton fiber: an integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose 27:8527–8550. https://doi.org/10.1007/s10570-020-03361-w
Correia J, Mathur K, Bourham M et al (2021) Surface functionalization of greige cotton knitted fabric through plasma and cationization for dyeing with reactive and acid dyes. Cellulose 28:9971–9990. https://doi.org/10.1007/s10570-021-04143-8
Farrell MJ, Ankeny MA, Hauser PJ (2014) Prediction of recipes for cotton cationisation and reactive dyeing to shade match conventionally dyed cotton. Color Technol 130:363–367. https://doi.org/10.1111/cote.12101
Goclik V, Stach S, Mischnick P (2004) Determination of (2,3-epoxypropyl)trimethylammonium chloride, (3-chloro-2-hydroxypropyl)trimethylammonium chloride, and (2,3-dihydroxypropyl) trimethylammonium chloride by capillary electrophoresis. J Chromatogr A 1038:283–289. https://doi.org/10.1016/j.chroma.2004.03.010
Haque ANMA, Hannan MA, Masud Rana M (2015) Compatibility analysis of reactive dyes by exhaustion-fixation and adsorption isotherm on knitted cotton fabric. Fash Text 2(1):1–12
Hashem MM (2006) Development of a one-stage process for pretreatment and cationisation of cotton fabric. Color Technol 122:135–144. https://doi.org/10.1111/j.1478-4408.2006.00022.x
Hashem A, El-Shishtawy RM (2001) Preparation and characterization of cationized cellulose for the removal of anionic dyes. Adsorpt Sci Technol 19:197–210. https://doi.org/10.1260/0263617011494088
Hashem M, Hauser P, Smith B (2003) Reaction efficiency for cellulose cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Text Res J 73:1017–1023
Hirosawa S, Minato K, Nakatsubo F (2001) Influence of carboxyl group on the acid hydrolysis of cellulose. J Wood Sci 47:141–144. https://doi.org/10.1007/BF00780563
Hsani M, Westman G, Potthast A, Rosenau T (2009) Cationization of cellulose by using N-oxiranylmethyl-N-methylmorpholinium chloride and 2-oxiranylpyridine as etherification agents. J Appl Polym Sci 114:1449–1456. https://doi.org/10.1002/app
Kanik M, Hauser PJ (2002) Printing of cationised cotton with reactive dyes. Color Technol 118:300–306. https://doi.org/10.1111/j.1478-4408.2002.tb00114.x
Kunze M, Karagiozidis P (1999) Levafix® Remazol®-high performance reactive dyes for all requirements and processes. DyStar Textilfarben GmbH&Co, Frankfurt
Li Y, Zhai S, Dong W et al (2022) Preparation of cationic viscose and its salt-free dyeing using reactive dye. Color Technol 138:378–387. https://doi.org/10.1111/cote.12598
Nallathambi A, Venkateshwarapuram Rengaswami GD (2016) Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr Polym 152:1–11. https://doi.org/10.1016/j.carbpol.2016.06.087
Pruś S, Kulpiński P, Matyjas-Zgondek E, Wojciechowski K (2022) Eco-friendly dyeing of cationised cotton with reactive dyes: mechanism of bonding reactive dyes with CHPTAC cationised cellulose. Cellulose 29:4167–4182. https://doi.org/10.1007/s10570-022-04521-w
Seemork K, Setthayanond J, Suwanruji P, Tooptompong P (2020) Utilization of spent coffee ground oil in eco-friendly scouring and reactive dyeing processes for cotton. Mater Sci Forum 1005:85–92. https://doi.org/10.4028/www.scientific.net/MSF.1005.85
Seemork K, Setthayanond J, Suwanruji P, Tooptompong P (2021) Optimization of salt-free reactive dyeing process for cotton with coconut oil/water dual-phase dyeing system. Asian J Chem 33:2099–2104. https://doi.org/10.14233/ajchem.2021.23300
Široký J, Blackburn RS, Bechtold T et al (2010) Attenuated total reflectance Fourier-transform infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 17:103–115. https://doi.org/10.1007/s10570-009-9378-x
Turcanu A, Bechtold T (2017) Cathodic decolourisation of reactive dyes in model effluents released from textile dyeing. J Clean Prod 142:1397–1405. https://doi.org/10.1016/j.jclepro.2016.11.167
Wang L, Xie G, Mi X et al (2022) A single-step pad-steam cationisation and dyeing process for improving dyeing properties of cotton fabrics. Color Technol 138:509–521. https://doi.org/10.1111/cote.12608
Zhang C, Ren J, Ma Y, Liu Y, Tang Y, Qin S (2018) Preparation and adsorption properties of amphoteric viscose fiber. Iran Polym J 27:635–644
Zhang T, Zhang S, Qian W et al (2021) Reactive dyeing of cationized cotton fabric: the effect of cationization level. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.1c04340
Acknowledgments
Financial support is gratefully acknowledged to the COMET Project “Textile Competence Center Vorarlberg 2—FFG 882502”, funded within COMET—Competence Centers for Excellent Technologies—by BMK, BMDW as well as co-financing federal province Vorarlberg. The COMET-Funding Program is managed by the Austrian Research Promotion Agency FFG.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. Financial support is gratefully acknowledged to the COMET Project “Textile Competence Center Vorarlberg 2—FFG 882502”, funded within COMET—Competence Centers for Excellent Technologies—by BMK, BMDW as well as co-financing federal province Vorarlberg. The COMET-Funding Program is managed by the Austrian Research Promotion Agency FFG. This work was financially supported by the Office of the Ministry of Higher Education, Science, Research and Innovation; and the Thailand Science Research and Innovation through the Kasetsart University Reinventing University Program 2021.
Author information
Authors and Affiliations
Contributions
JS: Funding acquisition, Supervision; FN: Investigation, Data collection and Analysis Writing first draft; KS: Investigation, Data collection and Analysis, Writing first draft; PS: Review and Editing; TB: Conceptualisation, Writing, Review and Editing; TP: Supervision, Funding acquisition; AM: Methodology, Writing Original draft, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have seen and approved the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Setthayanond, J., Netzer, F., Seemork, K. et al. Low-level cationisation of cotton opens a chemical saving route to salt free reactive dyeing. Cellulose 30, 4697–4711 (2023). https://doi.org/10.1007/s10570-023-05136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05136-5