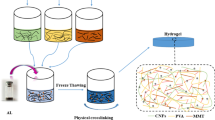
Abstract
Cellulose nanomaterial (CNM) and polyethylenimine (PEI) composites have attracted growing attention due to their multifunctional characteristics, which have been applied in different fields. In this work, soybean hulls were valorized into carboxyl cellulose nanofibrils (COOH-CNFs), and composited into hydrogels with PEI by combining them with cationic chelating and physical adsorption strategies. Cellulose nanofibrils (CNFs) were produced from soybean hulls prior to oxidation by a TEMPO mediated reaction to obtain COOH–CNFs; then drops of zinc chloride were added to 1.5% aqueous COOH–CNF dispersions, which were left for 24 h to form COOH-CNF hydrogels. Finally, the hydrogels were functionalized using different concentration of PEI solutions over a range of pH values. Elemental analysis results showed that 20% aq. PEI at pH 11.6 is the optimum condition to synthesize the COOH–CNF/PEI hydrogels. Additionally, the adsorption efficiency for the removal of anionic methyl blue dyes and Cu(II) ions from water was tested, reaching 82.6% and 69.8%, respectively, after 24 h. These results demonstrate the great potential of COOH–CNF/PEI hydrogels as adsorbent materials for water remediation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose nanomaterial/polyethylenimine (CNM-PEI) composites have attracted growing attention due to their multifunctional characteristics. Extensive research has been devoted to the design and development of new materials for different applications, such as paper making (Köklükaya al. 2018), wastewater treatment (Ge et al. 2016; Huang et al. 2020; Hong and Ryu 2021; Luo et al. 2022), drug release (Li et al. 2019; He et al. 2020; Wang et al. 2022), sensing (Jia et al. 2016; Mi et al. 2018), heterogeneous catalysis (Riva et al. 2020, 2021, 2022), and – of particular interest to this group – for water remediation.
Current research mainly focuses on synthesizing adsorbents derived from CNM-PEI composites by chemical crosslinking (Melone et al. 2015; Tang et al. 2015; Riva et al. 2021). The most frequently used crosslinking agents are glutaraldehyde (GAL) and epichlorohydrin (EPI), both of which have been reported to be highly toxic to humans and animals, causing second-hand contamination within water systems and the environment (Zeiger et al. 2005; Baselt et al. 2014; Zhang et al. 2017; Guo et al. 2018). Similarly, γ-(2,3-epoxypropoxy)propyltrimethoxysilane (GPTMS) and tri-functional trimethylolpropane-tris-(2-methyl-1-aziridine) propionate (TMPTAP) (Mo et al. 2019) have been used to synthesize CNM-PEI composites in recent years (Gan et al. 2019).
Considering the second-hand pollution issue caused by chemical crosslinkers, some research has focused on seeking a way to directly promote the crosslinking between nanocellulose and PEI without the need for additional chemical agents. The intermolecular interactions between the hydroxyl groups on the nanocellulose and the PEI amino groups is rather weak, which makes the formation of stable complexes by electrostatic interactions between these two motifs challenging. Therefore, pre-functionalization of the nanocellulose is preferred in order to either strengthen the intermolecular interactions that facilitate composite formation, or to provide appropriate functional groups that can be leveraged for covalent bonding to the PEI amino groups (Riva et al. 2021). The most popular pre-functionalized strategy is TEMPO-mediated oxidation, which can selectively introduce a carboxyl groups on the C-6 carbinol of the glucose residues in nanocellulose (Lal et al., 2019; Gomez-Maldonado et al. 2019). This group can then be used for covalent bond formation with the PEI amines via an amide linkage or facilitate the formation of ionic interactions (i.e. ammonium carboxylate formation) under appropriate conditions (Riva et al. 2021).
At present, heating and adding condensation agents to promote the combination of COOH-CNFs and PEI has been the most studied method (Riva et al. 2021). However, these processes require temperature and dialysis, making the overall procedure energy-and time-consuming. Another challenge for the use of CNM-PEI as an adsorbent is that most CNM-PEI composites exist in the form of a powder or aerogel. Their main drawbacks are that powders have poor separability and recyclability, while aerogels need high energy demand to generate absorbents. Hydrogels are a alternative material that seem to overcome the shortcomings of the powders and aerogels.
Regarding the raw material utilized to produce the composites, soybean hulls are an appealing alternative as a cellulose source. In the U.S., over 96 million tons of soybeans are produced annually (FAOSTAT 2021). Approximately 6–10% of the crop is a fibrous byproduct denominated soybean hull, which is traditionally used as cattle feed (Cassales et al. 2011; Hernandez et al. 2022). Previous work has demonstrated that soybean hulls can be valorized as bionanomaterial (FAOSTAT 2021; Hernandez et al. 2022). Soybean hulls contain variable amounts of cellulose (29–51%), hemicelluloses (10–25%), lignin (1–4%), pectins (4–8%), proteins (11–15%), soy oils (< 2.5%) along with other minor extractives (Liu et al., 2017). These components are easily extracted, and their properties are suitable for producing different materials. Nanofibrils of cellulose generated from soybean hulls exhibit high surface area, colloidal stability, and favorable polyanionic characteristics (Iglesias et al. 2021).
This work aims to address the design of stable hydrogels from soybean hulls and PEI. The hypothesis is that CNM-PEI composites can be synthesized by combing cationic chelation and physical adsorption. The novelty of this work relies on utilizing an agricultural by-product – soybean hulls – as a readily available raw material to produce hydrogels. To this end, TEMPO-oxidized CNFs from soybean hulls were chelated using zinc chloride at room temperature to form COOH-CNF hydrogels, then immersed into a PEI solution to achieve further functionalization by physical adsorption. In order to increase the amount of the absorbed PEI in the COOH-CNF hydrogel system, the effect of concentration and pH of PEI solutions was also investigated. In addition, the adsorption efficiency of the hydrogels for the capture of methyl blue dyes and Cu(II) ions from water was tested to explore the potential application of this new material for water remediation.
Materials and methods
Materials
CNF from bleached soybean hull fibers was produced at the Forest Products Development Center (Auburn, AL) by mechanical processing on a Masuko supermasscolloider (MKZA10-15 J, Masuko Sangyo Co., Fiber, Japan). For the preparation of the CNFs, bleached soybean hulls were diluted to a 2 wt% suspension. For the washing step, the suspensions were taken to a pH 3 using 1 M HCl solution, followed by a rinsing step using DI water until pH increased to 4.5. Then, NaHCO3 was added to obtain a 0.001 M concentration and pH was adjusted to 9 using 1 M NaOH for 30 min. Finally, several washings were performed until no changes in conductivity were measurable. The washed hulls were then processed by Masuko super mass colloider (MKZA-10–15 J) with 12 passes, obtaining a final consistency of 2.12 wt% at a pH of 5.8. Polyethylenimine (branched, Mw = 25,000 g/mol) was purchased from Sigma-Aldrich. (2,2,6,6-Tetramethylpiperidin-1-yl) oxyl (TEMPO) and methyl blue anionic (MB) dye was purchased from VWR, and the anhydrous copper (II) sulfate (98%) from Alfa Aesar.
TEMPO oxidation of CNF
TEMPO mediated oxidation of CNFs (COOH-CNFs) was adapted from studies previously reported (Lu et al. 2020). Briefly, 5 g of CNFs (dry mass basis) were suspended in 500 mL of distilled water containing TEMPO 0.08 g (0.1 mmol/g CNF) and NaBr 0.5 g (1 mmol/g CNF), and the pH was adjusted to 10 by addition of 0.5 M aq. NaOH. Then 30 mL of 12.5% aq. NaClO solution was added slowly to the CNF slurry (1.65 mL/10 min, total 3 h) and the reaction mixture was maintained at pH 10.5 at room temperature by adding aq. 0.5 M NaOH simultaneously. Furthermore, the oxidized CNFs were precipitated by adding ethanol (1500 mL) and then centrifuged at 3800 rpm. Finally, the oxidized CNFs were washed thoroughly with distilled water and filtered using a 0.20 µm cellulose acetate filter paper.
COOH-CNFs hydrogel formation and functionalization with PEI
COOH-CNF hydrogels were prepared by the dropwise addition of a 20 wt% aq. ZnCl2 solution along the walls of the glass tube into a 1.5 wt% aq. COOH-CNF dispersion. The final zinc ion concentration was controlled to be 0.1 mol/L (0.35 mL of 20% ZnCl2 solution). Then, the mixture was allowed to stand for 24 h without stirring to enable the formation of the nanocellulose hydrogels. Afterward, unbound Zn2+ ions were removed by rinsing the resulting hydrogels three times with 150 mL distilled water (Lu et al. 2020).
Next, COOH-CNF hydrogels were immersed in an aq. PEI solution at room temperature for 24 h to functionalize. The effects of pH (5–12) and concentration (2%, 5%, 10%, 20% and 40%) of PEI on the amount of PEI adsorbed in COOH-CNF hydrogel was investigated. Finally, the functionalized hydrogels were first placed into an 150 mL anhydrous ethanol solution for 2 h and then washed with 150 mL distilled water for about 5 times until the pH of the cleaning water remained neutral.
Characterization
Fourier transform infrared spectroscopy (FTIR)
Samples were freeze-dried at –50 °C for 48 h and then analyzed using a PerkinElmer Spotlight 400 FT-IR Imaging System (Massachusetts). An attenuated total reflectance (ATR) accessory with diamond/ZnSe crystal was used for collecting 64 scans with 4 cm−1 resolution. Data was processed with Spectrum 6 Spectroscopy Software (PerkinElmer).
Atomic force microscopy (AFM)
Surface characterization of the CNFs and COOH-CNFs were performed using Tosca 400 equipment from Anton-Paar (Gratz, Austria). Surfaces were prepared by spin-coating on PEI coated silicon wafers (2 × 2 cm2, with 150 µL of 0.01% liquid samples at 3000 rpm for 30 s). Height images were obtained by tapping mode with a NanoWorld (Neuchâtel, Switzerland) ARROW-NCR-20 silicon SPM-sensor cantilever with a resonance frequency of 285 kHz and constant force of 42 N/m; scan sizes were 5 × 5 µm2. Processing of the images was done with Gwyddion software 2.49 (SourceForge) and roughness calculations were done with ProfilmOnline (KLA Corporation, Milpitas, CA, U.S.).
Carboxyl group titration
The carboxyl content of oxidized cellulose samples was determined by conductometric titrations. The method was modified on the basis of previous studies (Saito et al. 2009). Briefly, 30 mg (dry basis) of COOH-CNF samples were suspended into 7.5 mL of 0.01 M HCl solution, and adjusted to a pH of 2. After 2 h of stirring, the suspensions were titrated with 0.01 M NaOH.
Dynamic light scattering (DLS)
The Zeta potential for 0.1% COOH-CNF and PEI solutions were tested on a pH range from 2 to 12. All measurements were done in a Litesizer from Anton-Paar (Gratz, Austria). For all samples, twelve repetitions were done and averaged.
Elemental analysis
Elemental analysis was performed on each sample using an elemental analyzer (Elementar vario MICRO, Ronkonkoma, NY, USA). A 2 mg sample of freeze-dried material was used for the test, and measurements were performed by triplicate.
Scanning electron microscopy (SEM)
Freeze-dried samples were set onto aluminum studs and sputtered with gold for 90 s in an EMS × 550 Sputter Coating Device from Science Services (Munich, Germany). Images with magnifications of × 50, × 100, and × 1000 were recorded in a Zeiss Evo 50VP SEM (Oberkochen, Germany).
Quartz crystal microbalance with dissipation monitoring (QCM-D)
All the experiments were performed on a Q-Sense analyzer (Biolin Scientific. Västra Frölunda, Sweden). These measurements were performed on gold sensors that were purchased from Biolin Scientific (Västra Frölunda, Sweden). Analyte solutions at a concentration of 0.1% were prepared and flowed onto the sensors at 100 µL/min at 25 °C, according to previous works (Gomez‐Maldonado et al., 2021).
Adsorption tests of methyl blue and Cu(II) ions
The adsorption efficiency of the hydrogels for methyl blue dyes and Cu (II) ions was tested. The hydrogels were cut into a 3.5 mm long cylinder (0.0186 g dry basis) and then placed into 10 mL of 200 mg/L methyl blue buffer solutions with pH 5.7 and left without shaking; aliquots were taken after different time intervals (3, 6, 12, 24 h) and the methyl blue dye concentrations in the supernatant were calculated using UV spectroscopy at 596 nm. Hydrogel cylinders of the same size were also put into 10 mL of 320 mg/L Cu(II) solutions for 24 h. The remaining Cu(II) ions in solution after hydrogel treatment were quantified by inductively coupled plasma mass spectrometry (ICP-MS).
Results and discussion
Characterization of CNFs and COOH-CNFs from soybean hulls
In order to confirm that the carboxyl group was successfully introduced to the CNFs, a FTIR analysis was carried out. The FTIR spectra of CNFs and COOH-CNFs are shown in Fig. 1A. The characteristic bands for CNFs and COOH-CNFs are observed at 3334 cm−1 and 2904 cm−1 which relate to O–H stretching and asymmetric C-H stretching, respectively. The band at 1723 cm−1 indicates a carboxyl stretching which is only observed in the COOH-CNF spectra, suggesting the formation of carboxylate after TEMPO mediated oxidation (Salama et al. 2015; Li et al. 2018). This was further confirmed by titration measurement where the carboxyl group content in the COOH-CNFs was 1.8–2.0 mmol/g CNF, similar to prior studies reported in literature (Qiu et al. 2014). The morphology of the fibers was assessed by AFM measurements, and the images of CNFs and COOH-CNFs are shown in Fig. 1B, C. After oxidation, the root mean square height (RSq) and arithmetic mean height (RSa) of the fiber decreased by 64% and 54.5%, which could be related to the decrease in fiber particle size. As can be observed in Fig. 1B, C, the CNF sample presents longer fibers compared with COOH-CNFs where the particles are clearly smaller, indicating a reduction in particle size after TEMPO-mediated oxidation (Lu et al. 2020).
Synthesis of hydrogels
To overcome the issues caused by chemical crosslinking, heating, and the use of condensation agents, a hydrogel absorbent was produced by combining cationic chelating and physical crosslinking. To achieve this strategy, TEMPO-oxidized CNF suspensions were chelated by zinc chloride at room temperature to form COOH-CNF hydrogels (Fig. 2B), then placed into PEI solutions to further functionalize by hydrogen bonding and electrostatic interactions, based on the hydroxyl group rich surface of the CNFs (Fig. 2C). The results showed that the hydrogels become opaque and appeared to be more rigid after functionalization by PEI, which is an indicator of successful adsorption of PEI into the COOH-CNF hydrogels.
Optimization on PEI adsorption conditions
The concentration and pH of the PEI solutions utilized to functionalize the hydrogels can impact their composition and performance. A first study was performed by varying the pH of PEI solutions using a reference concentration of 20% (Mo et al. 2019). Elemental analysis was utilized to confirm the presence of PEI in the COOH-CNF hydrogel system (Fig. 3A, D). The nitrogen content increased from 6.6% to 9.0% as the pH of the solution was increased from 5.1 to 11.7 (Fig. 3A), indicating that more PEI is absorbed in the hydrogel when the pH increased. It is important to emphasize that PEI can be adsorbed in the hydrogel system by means of hydrogen bonding, electrostatic interaction, and pore trapping (Yap et al. 2021). At pH values higher than 10, electrostatic repulsions occurs as evidenced by zeta potential values (Fig. 3B, C), causing PEI to desorb from the system. However, results show that the nitrogen content still increases when the pH exceeds 10 compared to what was found elsewhere (Hu et al. 2021). A possible explanation for this is that the amount of PEI adsorbed due to hydrogen bonding and pore trapping overcomes the desorption caused by the electrostatic repulsion at conditions of pH above 10 (Fig. 3B, C).
Further testing was done maintaining the adsorption pH at 11.65, where the adsorption of PEI was highest, while varying the PEI concentrations from 2 to 40%. Here, the nitrogen content increased with increasing concentration of PEI up to 20%, and then began to decline at higher PEI concentrations (Fig. 3D) as demonstrated by elemental analysis. This may be caused by the volume reduction of COOH-CNF/PEI hydrogel, as shown in Fig. 4F, leading to a decrease in surface area and pore size, which may be related to PEI immobilization. Thus, the best conditions for hydrogel synthesis were defined as pH 11.65 and 20% PEI concentration, resulting in 14.2% nitrogen content. A high nitrogen content in the hydrogel composite is essential for the adsorption ability of anionic dyes and copper ions, as will be discuss in the following section.
Characterization of hydrogels
FTIR was used to prove the nature of the interactions of the PEI molecules attached to the COOH-CNF hydrogels. The FTIR spectra of an unmodified COOH-CNF hydrogel, PEI, and COOH-CNF/PEI hydrogels are presented in Fig. 5A. The band at 1723 cm−1 corresponding to carboxyl groups was observed in the free COOH-CNF spectra, but cannot be found in the COOH-CNF hydrogel or the COOH-CNF/PEI hydrogels. This can be explained as complete chelation of the carboxyl group by zinc chloride, which should shift the asymmetric carboxyl stretching frequency to lower wavenumbers, thus obscuring it in the broader bands around 1600 cm−1 originating from cellulose and PEI (Lu et al. 2018). For PEI, the absorption at 2936 cm−1 and 2817 cm−1 were the characteristic bands of –CH2- stretching vibrations (Wang and Okubayashi 2019). The bands at 1670 cm−1 and 1050 cm−1 corresponded to the amino groups and C–C skeleton vibration, respectively (Guo et al. 2018; FAOSTAT 2021). Meanwhile, the absorbance band near 1599 cm−1 and 1459 cm−1 are the N–H bending vibration of primary amines in PEI (Wang et al. 2009; Wang et al. 2019). The absorption at 2936, 2817, and 1599 cm−1 were also found in the COOH-CNF/PEI hydrogels, which proves the successful introduction of PEI to COOH-CNF/PEI hydrogels. Moreover, no new bands were observed in the COOH-CNF/PEI hydrogels indicating that PEI functionalized hydrogel was physically blended and no chemical bonding occurred. Likewise, there was no notable change in the characteristic bands of PEI when the pH increases (Fig. 5B). However, when varying the PEI concentration from 2 to 40%, there is a slight difference in the N–H band at 1599 cm−1 (Fig. 5C). The value was the highest at 20% PEI concentration. This could be related to the higher PEI adsorption in the hydrogel at 20% PEI concentration, which is in agreement with the elemental analysis results.
Morphological characteristics of the hydrogels were studied by SEM. As shown in Fig. 6A, B, C, COOH–CNF hydrogels possessed a denser structure, compared with COOH-CNF/PEI hydrogels in Fig. 6D, E, F which showed an open and macroporous structure. A similar phenomenon was reported by Li et al. (2018), where CNF-PEI composite aerogels possess an open and macroporous honeycomb structure. While increasing surface area, the creation of pores within the sorbent network allows liquid to diffuse and fill the pores, which provides favorable interactions for the use of these materials for water contaminant removal (Yap et al. 2021). Key features of the pore structure including pore type, size, and volume determine the access and entrapment of the pollutants within the pores of the adsorbents (Wang et al. 2020; Yap et al. 2021).
QCM-D analysis
To better understand the interaction between COOH-CNFs and PEI, the adsorption phenomena was simulated on the surface of model films and followed by QCM-D at optimum conditions of pH and PEI concentration (Fig. 7 and Table 1). The chemicals used to synthesize the hydrogel were flowed through the QCM-D sensor in the same order that was used to prepare the hydrogels. The frequency and dissipation changes are shown in Fig. 7 and Table 1. A frequency shift of − 38.44 Hz was noted for the formation of the COOH-CNF film on the surface with a 3.56 ppm shift in dissipation, confirming the deposition of a viscous COOH-CNF film on the surface of the sensor, which was stable after pure water was flowed on top. When an aqueous solution of ZnCl2 was added, an increase in frequency of 1.22 Hz was observed as well as a dissipation drop of − 1.02 ppm. The negative shift in dissipation confirms chelation at the surface of the film (Alves et al. 2009), and the shift in frequency can be linked to water being released from the film after crosslinking. The change of pH from neutral to a more alkaline value (pH 11.65) induced swelling of the CNF film as more sodium ions were present in the media allowing for the weakening of the H-bonding (Junka et al. 2014) as indicated by frequency decreasing (− 11.63) and dissipation increasing (2.82). Finally, PEI was irreversibly adsorbed onto the surface of the crosslinked COOH-CNF model films as demonstrated by a negative frequency shift of − 27.16 Hz, that remaind attached after rinsing.
Hydrogel adsorption tests
For the cellulose-PEI composites, most research focus on adsorption of anionic dyes and metal ions, usually methyl blue and Cu(II) (Riva et al. 2021). Based on this, adsorption testing was done to quatify the removal ability of the hydrogels prepared in this study.
First, the effect of N content on the adsorption efficiency of methyl blue dye was tested to confirm the hypothesis that more PEI would result in a higher adsorption efficiency. As expected, the adsorption efficiency increased with the increase of the N content (Fig. 8B).
Based on the previous results, testing was done at 24 h adsorption under the optimal conditions (pH 5.7) for the methyl blue and Cu (II) ions adsorption. Results showed that the adsorption efficiency reached 82.6% and 69.8%, respectively (Fig. 9).
Conclusion
In this work, a COOH-CNF/PEI hydrogel originating from valorized soybean hulls was successfully synthesized by a combination of cationic chelation and physical adsorption. In order to increase the quantity of PEI on the surface, the effect of concentration and pH of the PEI solution on the N content of the composite was explored by elemental analysis. The results showed that the best conditions to introduce the highest nitrogen content (14.2%) in the COOH-CNF/PEI hydrogel were the combination of a 20% solution of PEI with a pH of 11.65. The adsorption efficiency of COOH-CNF/PEI hydrogels for the removal of methyl blue dye and Cu (II) from water were explored. The results showed that the adsorption efficiency for the methyl blue dyes and Cu (II) were 82.6% and 69.8%, respectively, after 24 h at pH 5.7. The results suggest that these hydrogel composites originating from soybean hulls have potential uses in water remediation applications.
Data availability
Data is available upon reasonable request to authors.
References
Alves NM, Picart C, Mano JF (2009) Self assembling and crosslinking of polyelectrolyte multilayer films of chitosan and alginate studied by QCM and IR spectroscopy. Macromol Biosci 9(8):776–785. https://doi.org/10.1002/mabi.200800336.[42]
Baselt R (2014) Encyclopedia of toxicology. J Anal Toxicol. https://doi.org/10.1093/jat/bku065
Cassales A, de Souza-Cruz PB, Rech R, Ayub MAZ (2011) Optimization of soybean hull acid hydrolysis and its characterization as a potential substrate for bioprocessing. Biomass Bioenergy 35(11):4675–4683. https://doi.org/10.1016/j.biombioe.2011.09.021
FAOSTAT (2021) Soybean. https://www.fao.org/faostat/en/#search/Soybeans. Accessed 29 Mar 2022.
Gan M, Yang G, Wang Z, Sui X, Hou Y (2019) Highly efficient oxidative desulfurization catalyzed by a polyoxometalate/carbonized cellulose nanofiber composite. Energy Fuels 34(1):778–786. https://doi.org/10.1021/acs.energyfuels.9b03712
Ge H, Huang H, Xu M, Chen Q (2016) Cellulose/poly (ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 23(4):2527–2537. https://doi.org/10.1007/s10570-016-0973-3
Gomez-Maldonado D, Vega Erramuspe IB, Peresin MS (2019) Natural polymers as alternative adsorbents and treatment agents for water remediation. BioResour 14(4):10093–10160. https://doi.org/10.15376/biores.14.4.Gomez-Maldonado
Gomez-Maldonado D, Filpponen I, Johansson LS, Waters MN, Vega Erramuspe IB, Peresin MS (2021) Environmentally dependent adsorption of 2, 4-dichlorophenol on cellulose-chitosan self-assembled composites. Biopolymers 112(8):e23434. https://doi.org/10.1002/bip.23434
Guo DM, An QD, Li R, Xiao ZY, Zhai SR (2018) Ultrahigh selective and efficient removal of anionic dyes by recyclable polyethylenimine-modified cellulose aerogels in batch and fixed-bed systems. Colloids Surf A Physicochem Eng Asp 555:150–160. https://doi.org/10.1016/j.colsurfa.2018.06.081
He H, Cheng M, Liang Y, Zhu H, Sun Y, Dong D, Wang S (2020) Intelligent cellulose nanofibers with excellent biocompatibility enable sustained antibacterial and drug release via a pH-responsive mechanism. J Agric Food Chem 68(11):3518–3527. https://doi.org/10.1021/acs.jafc.9b06588
Hernandez JA, Soni B, Iglesias MC, Vega Erramuspe IB, Frazier CE, Peresin MS (2022) Soybean hull pectin and nanocellulose: tack properties in aqueous pMDI dispersions. J Mater Sci 57(8):5022–5035. https://doi.org/10.1007/s10853-022-06938-x
Hong HJ, Ryu J (2021) Synthesis of copper nanoparticles from Cu2+-spiked wastewater via adsorptive separation and subsequent chemical reduction. Nanomaterials 11(8):2051. https://doi.org/10.3390/nano11082051
Hu D, Zhang T, Zhang S, He J, Dong X (2021) Diffusion of polyethyleneimine with different molecular weights into cotton fibers at low concentration. Cellulose 28(7):3997–4008. https://doi.org/10.1007/s10570-021-03795-w
Huang X, Li B, Wang S, Yue X, Zhengguo Y, Deng X, Ma J (2020) Facile in-situ synthesis of PEI-Pt modified bacterial cellulose bio-adsorbent and its distinctly selective adsorption of anionic dyes. Colloids Surf A Physicochem Eng Asp 586:124163. https://doi.org/10.1016/j.colsurfa.2019.124163
Iglesias MC, Hamade F, Aksoy B, Jiang Z, Davis VA, Peresin MS (2021) Correlations between rheological behavior and intrinsic properties of nanofibrillated cellulose from wood and soybean hulls with varying lignin content. BioResources 16(3):4831. https://doi.org/10.15376/biores.16.3.4831-4845
Jia Y, Yu H, Zhang Y, Dong F, Li Z (2016) Cellulose acetate nanofibers coated layer-by-layer with polyethylenimine and graphene oxide on a quartz crystal microbalance for use as a highly sensitive ammonia sensor. Colloids Surf B 148:263–269. https://doi.org/10.1016/j.colsurfb.2016.09.007
Junka K, Guo J, Filpponen I, Laine J, Rojas OJ (2014) Modification of cellulose nanofibrils with luminescent carbon dots. Biomacromol 15(3):876–881. https://doi.org/10.1021/bm4017176.[43]
Köklükaya O, Carosio F, Wågberg L (2018) Tailoring flame-retardancy and strength of papers via layer-by-layer treatment of cellulose fibers. Cellulose 25(4):2691–2709. https://doi.org/10.1007/s10570-018-1749-8
Lal SS, Mhaske ST (2019) TEMPO-oxidized cellulose nanofiber/kafirin protein thin film crosslinked by Maillard reaction. Cellulose 26(10):6099–6118. https://doi.org/10.1007/s10570-019-02509-7
Li J, Zuo K, Wu W, Xu Z, Yi Y, Jing Y, Dai H, Fang G (2018) Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr Polym 196:376–384. https://doi.org/10.1016/j.carbpol.2018.05.015
Li N, Zhang H, Xiao Y, Huang Y, Xu M, You D, Yu J (2019) Fabrication of cellulose-nanocrystal-based folate targeted nanomedicine via layer-by-layer assembly with lysosomal pH-controlled drug release into the nucleus. Biomacromol 20(2):937–948. https://doi.org/10.1021/acs.biomac.8b01556
Liu HM, Li HY (2017) Application and conversion of soybean hulls. Soybean-the basis of yield, biomass and productivity. IntechOpen. https://doi.org/10.5772/66744
Lu P, Liu R, Liu X, Wu M (2018) Preparation of self-supporting bagasse cellulose nanofibrils hydrogels induced by zinc ions. Nanomaterials 8(10):800. https://doi.org/10.3390/nano8100800
Lu P, Yang Y, Liu R, Liu X, Ma J, Wu M, Wang S (2020) Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr Polym 249:116831. https://doi.org/10.1016/j.carbpol.2020.116831
Luo T, Wang R, Chai F, Jiang L, Rao P, Yan L, Hu X, Zhang W, Wei L, Khataee A, Han N (2022) Arsenite (III) removal via manganese-decoration on cellulose nanocrystal-grafted polyethyleneimine nanocomposite. Chemosphere 303:134925. https://doi.org/10.1016/j.chemosphere.2022.134925
Melone L, Rossi B, Pastori N, Panzeri W, Mele A, Punta C (2015) TEMPO-oxidized cellulose cross-linked with branched polyethyleneimine: nanostructured adsorbent sponges for water remediation. ChemPlusChem 80(9):1408–1415. https://doi.org/10.1002/cplu.201500145
Mi HY, Jing X, Zheng Q, Fang L, Huang HX, Turng LS, Gong S (2018) High-performance flexible triboelectric nanogenerator based on porous aerogels and electrospun nanofibers for energy harvesting and sensitive self-powered sensing. Nano Energy 48:327–336. https://doi.org/10.1016/j.nanoen.2018.03.050
Mo L, Pang H, Tan Y, Zhang S, Li J (2019) 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh efficient and reversible removal of Cu (II) ions from water. Chem Eng J 378:122157. https://doi.org/10.1016/j.cej.2019.122157
Qiu B, Guo J, Zhang X, Sun D, Gu H, Wang Q, Wang H, Wand X, Zhang X, Weeks BL, Guo Z, Wei S (2014) Polyethylenimine facilitated ethyl cellulose for hexavalent chromium removal with a wide pH range. ACS Appl Mater Interfaces 6(22):19816–19824. https://doi.org/10.1021/am505170j
Riva L, Punta C, Sacchetti A (2020) Co-polymeric nanosponges from cellulose biomass as heterogeneous catalysts for amine-catalyzed organic reactions. ChemCatChem 12(24):6214–6222. https://doi.org/10.1002/cctc.202001157
Riva L, Fiorati A, Punta C (2021) Synthesis and application of cellulose-polyethyleneimine composites and nanocomposites: a concise review. Materials 14(3):473. https://doi.org/10.3390/ma14030473
Riva L, Lotito AD, Punta C, Sacchetti A (2022) Zinc-and Copper-loaded nanosponges from cellulose nanofibers hydrogels: new heterogeneous catalysts for the synthesis of aromatic acetals. Gels 8(1):54. https://doi.org/10.3390/gels8010054
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromol 10(7):1992–1996. https://doi.org/10.1021/bm900414t
Salama A, Shukry N, El-Sakhawy M (2015) Carboxymethyl cellulose-g-poly (2-(dimethylamino) ethyl methacrylate) hydrogel as adsorbent for dye removal. Int J Biol Macromol 73:72–75. https://doi.org/10.1016/j.ijbiomac.2014.11.002
Tang XZ, Yu B, Hansen RV, Chen X, Hu X, Yang J (2015) Grafting low contents of branched polyethylenimine onto carbon fibers to effectively improve their interfacial shear strength with an epoxy matrix. Adv Mater Interfaces 2(12):1500122. https://doi.org/10.1002/admi.201500122
Wang C, Okubayashi S (2019) Polyethyleneimine-crosslinked cellulose aerogel for combustion CO2 capture. Carbohydr Polym 225:115248. https://doi.org/10.1016/j.carbpol.2019.115248
Wang X, Schwartz V, Clark JC, Ma X, Overbury SH, Xiaochun X, Song C (2009) Infrared study of CO2 sorption over “molecular basket” sorbent consisting of polyethylenimine-modified mesoporous molecular sieve. J Phys Chem C 113(17):7260–7268. https://doi.org/10.1021/jp809946y
Wang L, Shi C, Pan L, Zhang X, Zou JJ (2020) Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: a review. Nanoscale 12(8):4790–4815. https://doi.org/10.1039/C9NR09274A
Wang Q, Li M, Zheng Z, Niu Y, Xue X, Ao C, Zhang W, Lu C (2022) Polyethylenimine-functionalized nanofiber nonwovens electrospun from cotton cellulose for wound dressing with high drug loading and sustained release properties. Polymers 14(9):1748. https://doi.org/10.3390/polym14091748
Yap PL, Nine MJ, Hassan K, Tung TT, Tran DN, Losic D (2021) Graphene-based sorbents for multipollutants removal in water: a review of recent progress. Adv Funct Mater 31(9):2007356. https://doi.org/10.1002/adfm.202007356
Zeiger E, Gollapudi B, Spencer P (2005) Genetic toxicity and carcinogenicity studies of glutaraldehyde—a review. Mutat Res Rev Mutat Res 589(2):136–151. https://doi.org/10.1016/j.mrrev.2005.01.001
Zhang N, Zang GL, Shi C, Yu HQ, Sheng GP (2016) A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J Hazard Mater 316:11–18. https://doi.org/10.1016/j.jhazmat.2016.05.018
Zhang C, Su J, Zhu H, Xiong J, Liu X, Li D, Chen Y, Li Y (2017) The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv 7(54):34182–34191. https://doi.org/10.1039/C7RA03056H
Acknowledgments
The authors would like to acknowledge the Northeastern University Snell Library for covering the full APC of this article and their effort to make research accessible for everybody. The authors would also like to acknowledge Dr. Mehmet Zeki Billor from the Department of Geosciences at Auburn University for his help in the ICP-MS data acquisition.
Funding
Open access funding provided by Northeastern University Library. This work was supported by the National Science Foundation CAREER award (2119809) through the BMAT program in the Division of Materials Research and the EPSCoR program. Likewise, the USDA National Institute of Food and Agriculture, Hatch program (ALA013-17003), and McIntire-Stennis program (1022526) are acknowledged for the support of this work. The authors also want to acknowledge the support from Chinese Scholarship Council (201906300036).
Author information
Authors and Affiliations
Contributions
YN: Conceptualization, Methodology, Investigation, Formal Analysis, Visualizaiton, Writing—original draft; DG-M: Conceptualization, Methodology, Formal Analysis, Visualization, Writing—review & editing. MCI: Visualization, Writing—review & editing; DCW: Methodology, Writing—review & editing; MSP: Conceptualization, Methodology, Visualization, Supervision, Funding acquisition, Writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nan, Y., Gomez-Maldonado, D., Iglesias, M.C. et al. Valorized soybean hulls as TEMPO-oxidized cellulose nanofibril and polyethylenimine composite hydrogels and their potential removal of water pollutants. Cellulose 30, 3639–3651 (2023). https://doi.org/10.1007/s10570-023-05086-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05086-y