Abstract
The development of environmentally friendly, multifunctional conductive membranes is an ideal candidate for future new electronic components. Some cellulose-based electronic sensors have been studied, such as pressure sensors, hydrothermal sensors, flexible sensors, optoelectronic devices, and electrochemical energy storage, etc. However, there are very few studies on cellulose-based conductive materials in Joule heating. In this study, we adopted a simple and environmentally friendly silane crosslinking method to crosslink cellulose and graphene oxide (GO) together. The cellulose/GO hybrid membrane were characterized by FTIR, Raman, SEM, AFM, TGA, surface wettability, self-cleaning, surface resistance, Joule heating and other tests. Results showed that the covalent bond between cellulose and GO was formed by vinyltrimethoxysilane (VTMS), and GO was successfully cross-linked on the surface of the cellulose membrane. The cellulose/GO hybrid membrane has good thermal stability, strong hydrophobicity and self-cleaning properties. When the GO concentration was 3 w/w%, the Young's modulus of the film reached the maximum (47.38% higher than that of the original sample). In addition, it also exhibited extremely low surface resistivity (720.69 Ω), controllable Joule heating capability, extremely fast thermal response (heating process and cooling process within 5 s) and good electrothermal stability. It showed great potential in multi-functional electronic products such as electric heating electronic devices, electric heating sensors, and smart clothing in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent years, there has been great interest in developing small, multifunctional electronics, especially in sensor, wearable electronics and monitor fields (Han et al. 2022; Zhang et al. 2022b). Conventional electronic devices involve a large amount of non-renewable, refractory and low-durability polymers, which inevitably lead to waste of resources and pollution (Liu et al. 2020). Therefore, environmentally friendly, renewable, stable, easily processable and multifunctional conductive composites becomes an ideal candidate for future electronic components (Karimah et al. 2021; Cenci et al. 2022).
As we all know, cellulose has a wide range of sources that can be extracted from trees, leaves and crops, etc. Also, it has many advantages like low cost, high strength, stability, and degradability. At the same time, cellulose has abundant hydroxyl groups, which endows the possibility of chemical modification. However, cellulose is an insulator material, and how to impart electrical conductivity to cellulose-based composites is a hot topic in the scientific community (Liu et al. 2020).
Conductive carbon material such as carbon nanotube, graphite, graphene and graphene oxide (GO) are lightweight material with various structures and properties, thus facilitating large-scale applications, and has been used as a substitute for metals (Krifa 2021). Despite the limited conductivity, GO as a derivative of graphene has abundant hydroxyl, carboxyl and epoxy groups, which is easy for crosslinking.
Physical blending (Valentini et al. 2013; Pan et al. 2021), layer-by-layer stacking (Gao et al. 2013; Li et al. 2020), vacuum filtration (Hou et al. 2018), etc. are popular method to prepare conductive hybrids of cellulose and graphene and its derivatives by many studies. But, due to the weak van der Waals forces such as π-π bond and hydrogen bond between such carbon material powders, it is difficult to disperse them uniformly. Many attempts have been done to solve those problems. Ghulam Yasin et al. used surfactants to enhance the electrostatic repulsion of graphene and hinder the agglomeration. However, the introduction of new impurities led to a decrease in the interfacial bonding force between graphene and the polymer, which weakened the performance of the coating (Yasin et al. 2018). Gaojie Han et al. successfully developed cellulose-based Ni-decorated graphene magnetic film (Han et al. 2021). Firstly, they subjected Ni(OH)2 nanoribbon to hydrothermal reaction under high pressure for 12 h, followed by in-situ thermal reduction with GO suspension for 12 h, and finally vacuum filtration. It takes advantage of good dispersion of GO, but this work is time-consuming and complex.
Silane crosslinking by grafting of vinyl-silane to polymer chains and thermal curing, to create C–Si–O–Si–C bonds is also described (Goethals 2021; Salwa et al. 2022). Silane modification of GO perfectly solves its dispersion problem, and has a good layered structure to form a dense coating (Hou et al. 2010; Chen et al. 2022). Hemant Mittal successfully prepared GO/chitosan (CS)/carboxymethyl cellulose cross-linked nanocomposite hydrogels using vinyltriethoxysilane (VTES) for dye adsorption (Mittal et al. 2021). Y.Chen et al. utilized silane-modified GO to prepare a dense hydrophobic layer and explored its anti-corrosion properties (Chen et al. 2022). Kingshuk Dhali et al. modified cellulose by VTES and explored its surface properties (Dhali et al. 2022). Mandana Dilamian et al. prepared superhydrophobic and lipophilic cellulose aerogels with methyltrimethoxysilane for oil–water separation (Dilamian and Noroozi 2021).
However, these works mainly focus on exploring environmental remediation, physical and surface properties, etc. There are few studies on the crosslinking of cellulose composites in electronics.
In this work, we use an eco-friendly, simple and time-saving process for cellulose/GO crosslinking without high temperature, pressure and any toxic reagents. The results showed that cellulose was successfully cross-linked of cellulose and GO, with excellent thermal stability, good mechanical property, hydrophobicity, self-cleaning ability, low resistivity, great joule heating performance and quick response time. This method provides a new idea for peroxide crosslinking. The prepared samples have potential applications in various fields.
Experimental
Materials
Cellulose membrane (pore size 0.22 μm, weight 0.95 g), ethanol, acetone, hydrochloric acid (HCl) and vinyltrimethoxysilane (VTMS, 98%) were supplied by Sigma Aldrich (ST. Louis, USA). GO was provided by the Technical University of Liberec. All the solutions used distilled water with 18 MΩ cm electrical resistivity.
Preparation of silane-crosslinked cellulose/GO hbrid membrane
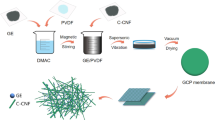
At first, 5 w/w% VTMS was added into 30 ml 80% ethanol solution at pH 4, which was adjusted by HCl, for 30 min for the hydrolysis of VTMS. Then, different concentration of GO (1 w/w %, 2 w/w %, 3 w/w %, 4 w/w %, 5 w/w %) and cellulose membrane were added into the above VTMS solution for crosslinking and stirred at low speed for 5 h at 80 °C. After the silane reaction, the membrane was taken out and dried in a vacuum oven at 120 °C for 10 min to promote cross-linking. Finally, samples were washed in ethanol and acetone solutions to remove unreacted GO and silane.
FTIR and Raman
The changes in functional group of samples was chemically investigated by FTIR spectrometer (Nicolet IZ10). Spectrum was obtained in the spectral region between 400 cm−1 and 4000 cm−1 with 2 cm−1 resolution.
Morphology
The surface morphology of the cellulose/GO hybrid membrane was observed by Scanning electron microscope (SEM) (VEGA TESCAN Inc., USA) and atomic force microscope (AFM).
Grafting density of cellulose/GO hybrid membrane
The grafting density (D %) was calculated by the increase of weight of cellulose/GO hybrid membrane after silane reaction following the equation (Anirudhan et al. 2018):
where \({W}_{a}\) is the dry weight of the composite after silane crosslinking and \({W}_{b}\) is the dry weight of the cellulose membrane before silane crosslinking. With the increase of GO concentration, the D % changes as shown in Fig. 1.
Thermal
5–10 mg sample was put into the aluminum film box and was measured by TGA/SDTA851 from 25 to 600 °C at a heating rate of 10 °C/min. The experiment was carried out under N2 gas at a flow rate of 10 mL/min.
Mechanical properties
Cellulose/GO Membrane was cut into 40 mm length and 3 mm width pieces, and their thickness (0.11 mm) was measured with a micrometer. Stress and strain tests were carried out by LaborTech TCM 151 at a speed of 50 mm/min.
Water contact angle (WCA)
The WCA measurements were carried out by optical contact angle meter, which was equipped with a special purpose software following ISO 27448:2009 test method. The measurements were performed at room temperature (25 °C) and 50–60% relative humidity. The sample was placed horizontally on the observation platform with double-sided tape, and the observation camera was observed perpendicular to the horizontal plane. Deionized water was dropped onto the surface of the samples from the tip of a micro-pipette (5 µL) and the image of the droplet was captured at each observation time point, the shape of the droplet was analyzed to determine the static contact angle. Generally, five drops were measured of each sample, and then the average value was calculated.
Resistivity of cellulose/GO hybrid membrane
The surface resistance of cellulose/GO hybrid membranes were tested by a4339B High Resistance Meter (Hewlett Packard Ohmmeter) according to the standard ASTM D257–07. During the test, a 0.1 V DC power supply and concentric electrodes were used at the pressure of 2.3 kPa, temperature T = 25.5 °C, and the relative humidity RH = 52%. Surface resistance was measured by putting a voltage potential between two electrodes that are in contract with the same side of the tested material. The surface resistance ρs was calculated from equation:
where \({\rho }_{s}\) is the surface resistivity (Ω); \({R}_{s}\) is surface resistance (Ω); \(o\) is the middle perimeter of electrodes (m) and \(l\) is the distance of electrode (m).
Joule heating property
Both sides of the cellulose/GO conductive hybrid membrane was fixed by electrode clips, and the distance between the electrodes at both ends is 3 cm. Then, DC power was connected and output different voltages (3 V, 6 V, 9 V, 12 V, 15 V). Changes of temperature were monitored by thermal camera.
Results
Characterization of cellulose/GO structure
To study the chemical interaction between cellulose and GO after silane crosslinking, original cellulose membrane and cellulose/GO hybrid membrane were selected for comparison. Figure 2 shows the comparison of FTIR spectra of cellulose membranes and cellulose/GO hybrid membranes. It can be clearly found that many new characteristic peaks appear in the cellulose/GO hybrid film after silane crosslinking. The new characteristic peaks around 1645 cm−1 correspond to the C=C and C=O stretching vibrations of GO (Chen et al. 2021). The characteristic peak observed at 1411 cm−1 is attributed to the stretching vibration of CH2 on VTMS. Also, the peak at 1276 cm−1 is due to the vibration of CH3 (Wu et al. 2017). The unique characteristic peak of cellulose can be observed around 1050 cm−1, which is attributed to the C–O–C stretching vibration of the glycosidic ring (Rafieian et al. 2018). However, a shoulder peak at 1008 cm−1 can be observed in the cellulose/GO spectra, confirming the presence of Si–O (Golova et al. 2020). The peaks appearing from 700 cm−1 to 850 cm−1 are attributed to Si–C bonds. The appearance of these peaks confirms that complete hydrolysis of VTMS achieving cellulose/GO cross-linking (Cruz-Aguilar et al. 2018). Interestingly, the OH stretching vibration of the cellulose/GO films weakened after silanization, which may be due to the complete hydrolysis of VTMS and the formation of condensation with the hydroxyl groups of GO and cellulose forming (Si–O)n chains (Wilamowska-Zawlocka et al. 2016).
Raman spectroscopy is widely used as an effective method to study the structure of graphene and its derivatives. Figure 3a shows the Raman spectroscopy results of cellulose/GO hybrid membrane and pristine GO powders. From the figure, we can clearly find the unique D, G and 2D characteristic bands of GO, indicating the successful grafting of GO to the surface of the cellulose membrane (Zhang et al. 2022a). The broad and symmetrical 2D peaks indicate the good dispersion of GO during the cross-linking process with the cellulose membrane surface (Chen et al. 2022). The D band is usually associated with the in-plane sp3 hybrid carbon irregularity, and the G band is ascribed to the in-plane vibrations of the sp2 hybrid carbon in the hexagonal lattice (Chen et al. 2021). At the same time, we noticed that after the silane reaction, the G band of the sample shifted to the lower wavelength, which verified the multilayer deposition of GO on the surface of the cellulose film. In addition, the D* band appears around 1100 cm−1, which may be related to the disordered graphite lattice according to the relevant literature (López-Díaz et al. 2020). The ratio of D peak to G peak intensities \(\left( {{\raise0.7ex\hbox{${I_{D} }$} \!\mathord{\left/ {\vphantom {{I_{D} } {I_{G} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${I_{G} }$}}} \right)\) was used as an effective means to evaluate defects in GO composites. From Fig. 3b, compared with pristine GO powder, \({{\raise0.7ex\hbox{${I_{D} }$} \!\mathord{\left/ {\vphantom {{I_{D} } {I_{G} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${I_{G} }$}}} \) value first decreased and then increased with increasing GO concentration. This may be due to the successful covalent reaction of GO with the silanol groups in VTMS at low GO concentrations without causing damage to the carbon lattice during the condensation process. There is also a possibility that, after the silane reaction, silane covers the surface defects present in the GO coating (Ahmadi et al. 2016; Raza et al. 2018). But this conclusion needs further confirmation. However, as the GO concentration increases, it can be observed that 2D band increase and broaden, indicating the multilayer coating of GO. At the same time, the increase of \({{\raise0.7ex\hbox{${I_{D} }$} \!\mathord{\left/ {\vphantom {{I_{D} } {I_{G} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${I_{G} }$}}} \) value indicates that too many GO powders were deposited on the surface of the cellulose film. Due to insufficient silanization and randomness of deposition, GO grown in different regions, which cannot guarantee a uniform crystal extension orientation, forming a larger defect.
Surface morphology of cellulose/GO hybrid membranes
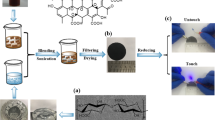
Figure 4 shows the surface morphological characteristics of pristine cellulose membrane and cellulose/GO hybrid membrane. From the figure, it can be seen that with the increase of GO concentration, the surface of cellulose film is gradually coated by GO to form a dense network and coarse surface. However, when the concentration further increased, it can be clearly seen from the image that excessive accumulation of GO forms multilayers, and a hole-like structure, which may have an impact on the surface roughness and electrical properties. The EDS image on the right demonstrates the presence of Si in the silane-crosslinked cellulose/GO hybrid film. Table 1 shows the content of C, O and Si element on cellulose/GO membrane surface. With the addition of more GO, the content of C and Si elements on the film surface increased, while the O element decreased indicating that GO powders were successfully cross-linked on cellulose surface by the condensation between VTMS and oxygen-containing groups. In addition, we investigated membrane surface roughness using AFM. From Fig. 5, as the GO concentration increased, more and more GO powders were cross-linked or deposited on the film, forming a rough surface, which Ra value increased from 126.4 nm to 244.4 nm. This result verifies that when the GO concentration is gradually increased, a rougher and irregular multilayer structure is formed on the film surface.
Thermal analysis of cellulose/GO hybrid membrane
Thermal analysis was used to investigate the stability of the samples at higher temperatures and the degradation behavior at extreme temperatures. As shown in Fig. 6, the cellulose membrane and the cellulose/GO hybrid membrane exhibited different patterns between 0 and 180 °C. Pure cellulose membranes lose weight slowly at this temperature due to the removal of moisture, hydroxyl or carboxyl groups from the cellulose. However, the cellulose/GO hybrid membrane showed nothing change. The possible reason is that after being cross-linked by silane, it turned into a hydrophobic film with no moisture present. The hydrophobicity of cellulose/GO hybrid membranes will be introduced in later section. Between 200 and 240 °C, both films lost weight rapidly, which may be related to the depolymerization, dehydration, and decomposition of cellulose sugar-based units (Tan et al. 2022). From the DTG curve on the right, it can be found that the maximum degradation temperature of the cellulose/GO film is higher than that of the cellulose film, indicating that the thermal stability of the hybrid film is improved after silane crosslinking.
Mechanical analysis
The mechanical properties of the hybrid films were also investigated. As shown in Fig. 7, with the increase of GO concentration, the tensile strength and Young's modulus of the hybrid films first increased and then decreased. Compared with the unmodified cellulose membrane, the Young's modulus of the cellulose/GO hybrid membrane reaches the maximum value (113.49 MPa) when the GO concentration is 3 w/w%, which is increased by 47.38%. On the one hand, this may be due to the existence of Si–O–Si and Si–C bonds in the composite after silane crosslinking, which strengthens the interfacial interaction between cellulose and GO, and improves the Young's modulus and tensile strength. On the other hand, as the GO concentration increased, more GO particles formed cross-links with the surface of the cellulose membrane, synergistically enhancing its mechanical property. But, when adding excessive GO, the strength of the hybrid films significantly reduced, which may be related to insufficient cross-linking or uneven dispersion of GO. When the hybrid film is affected by external force, the stress cannot be well and uniformly transferred to the reinforcing phase, resulting in the reduction of the stress. Results show that the silane-crosslinked cellulose/GO hybrid film improves the mechanical strength.
Surface wettability and self-cleaning of cellulose/GO hybrid membrane
Surface wettability of cellulose/GO hybrid membrane was investigated by WCA. As is shown in Fig. 8, The unmodified cellulose film quickly absorbed water droplets with no measurable WCA. In contrast, the silane-crosslinked cellulose/GO films exhibited high WCAs of 112.5°–126.7°, revealing their hydrophobicity and could be classified as a hydrophobic material. This may be related to the condensation of oxygen-containing functional groups on cellulose and GO with hydrophobic VTMS forming covalent bonds and the hydrophobic silane forms a protective layer on the surface, making the sample hydrophobic. In addition, the rough membrane surface also promotes its hydrophobicity. We used soot to smear the membrane surface to test its self-cleaning properties. As can be seen in Fig. 9b, compared with the pristine cellulose membrane, the soot on the cellulose/GO hybrid membrane is easily carried away by water droplets leaving a clean surface. This may be due to a hydrophobic layer on the membrane surface after silane crosslinking. The low surface energy makes it extremely difficult for impurities to adhere on the membrane surface, where they can be easily encapsulated and removed by water.
Electrical and Joule heating properties
Herein, we examine the electrical conductivity and Joule heating capability of cellulose/GO hybrid films. As shown in Fig. 9a, the cellulose/GO hybrid membrane had the lowest surface resistivity (720.69 Ω) when the GO content was 3 w/w%, revealing the great conductivity. At low GO loadings, discontinuous and isolated GO distributions coated on the membrane surface, implying a poor conductive network. When the GO loading is gradually increased, a continuous conductive network is formed, which is favorable for electron transport.
The good electrical conductivity endows the hybrid film with excellent Joule heating capability. Figure 9b shows the Joule heating capacity of the hybrid films at different GO concentrations. According to Joule’s law (\(\mathrm{Q}=\frac{{U}^{2}}{R}t\), Q, U, R, and t are heat generated, applied voltage, sample resistance, and operating time, respectively), samples with lower surface resistivity have extremely high Joule heating performance. The hybrid membrane exhibits a controllable heating temperature with changes of applied voltage. As shown in Fig. 9c, as the voltage increases, the heating temperature is higher. Under the safe voltage of 15 V, it can reach 182 °C. In addition, we can find that the heating process reaches a very high level within 5 s, indicating that its thermal response time is very fast. Likewise, the membrane surface temperature decreased rapidly within 5 s after the power was turned off, exhibiting excellent controllability. To test the stability of its Joule heating performance, we switched the voltage every 10 s and recorded its heating and cooling temperatures. As shown in Fig. 9d, in the switching power with repeated cycles, the heating temperature of the membrane fluctuates very little, revealing its stable electrical heating performance. The above results indicate that the good electrical conductivity and excellent Joule heating performance show great potential in various electronic fields such as electrothermal clothing, wearable devices, and sensor systems.
Conclusion
In this study, cellulose/GO multifunctional hybrid membranes were successfully prepared by silane crosslinking. The oxygen-containing groups on the surface of cellulose and GO condense with VTMS to form covalent bonds. Silane modification can improve their mechanical and electrical properties. The prepared hybrid membranes have good thermal stability, improved mechanical properties, excellent hydrophobicity, self-cleaning properties and low surface resistivity (720.69 Ω). Furthermore, it has excellent performance in Joule heating with controllable electric heating temperature under different voltages, extremely fast thermal response speed (within 5 s), and stable electrical performance by repeatedly switching the power supply. To sum up, this multifunctional membrane with hydrophobicity, self-cleaning and excellent electrical properties is a strong candidate for a new generation of electronic components.
Availability of data and materials
All of the material and data are owned by the authors and/or no permissions are required.
References
Ahmadi A, Ramezanzadeh B, Mahdavian M (2016) Hybrid silane coating reinforced with silanized graphene oxide nanosheets with improved corrosion protective performance. Rsc Adv 6:54102–54112
Anirudhan TS, Deepa JR, Binussreejayan (2018) Electrochemical sensing of cholesterol by molecularly imprinted polymer of silylated graphene oxide and chemically modified nanocellulose polymer. Mater Sci Eng C 92:942–956. https://doi.org/10.1016/J.MSEC.2018.07.041
Cenci MP, Scarazzato T, Munchen DD et al (2022) Eco-friendly electronics—a comprehensive review. Adv Mater Technol 7:2001263
Chen Y, Liu YW, Xie Y et al (2021) Preparation and anti-corrosion performance of superhydrophobic silane/graphene oxide composite coating on copper. Surf Coat Technol 423:127622. https://doi.org/10.1016/J.SURFCOAT.2021.127622
Chen Y, Liu YW, Xie Y et al (2022) Preparation of hydrophobic silane/graphene oxide composite coating implanted with benzotriazole to improve the anti-corrosion performance of copper. J Alloys Compd 893:162305. https://doi.org/10.1016/J.JALLCOM.2021.162305
Cruz-Aguilar A, Navarro-Rodríguez D, Pérez-Camacho O et al (2018) High-density polyethylene/graphene oxide nanocomposites prepared via in situ polymerization: morphology, thermal, and electrical properties. Mater Today Commun 16:232–241. https://doi.org/10.1016/J.MTCOMM.2018.06.003
Dhali K, Daver F, Cass P, Adhikari B (2022) Surface modification of the cellulose nanocrystals through vinyl silane grafting. Int J Biol Macromol 200:397–408. https://doi.org/10.1016/J.IJBIOMAC.2022.01.079
Dilamian M, Noroozi B (2021) Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr Polym 251:117016. https://doi.org/10.1016/J.CARBPOL.2020.117016
Gao K, Shao Z, Wu X et al (2013) Cellulose nanofibers/reduced graphene oxide flexible transparent conductive paper. Carbohydr Polym 97:243–251. https://doi.org/10.1016/J.CARBPOL.2013.03.067
Goethals R (2021) Crosslinking Technologies. Glob Cable Ind. 215–248
Golova LK, Makarov IS, Bondarenko GN et al (2020) Composite fibers based on cellulose and vinyltriethoxysilane as precursors of carbon materials. Polym Sci Ser B 62:152–162
Han G, Ma Z, Zhou B et al (2021) Cellulose-based Ni-decorated graphene magnetic film for electromagnetic interference shielding. J Colloid Interface Sci 583:571–578. https://doi.org/10.1016/J.JCIS.2020.09.072
Han Y, Ruan K, Gu J (2022) Janus (BNNS/ANF)-(AgNWs/ANF) thermal conductivity composite films with superior electromagnetic interference shielding and Joule heating performances. Nano Res 15:4747–4755. https://doi.org/10.1007/s12274-022-4159-z
Hou S, Su S, Kasner ML et al (2010) Formation of highly stable dispersions of silane-functionalized reduced graphene oxide. Chem Phys Lett 501:68–74. https://doi.org/10.1016/J.CPLETT.2010.10.051
Hou M, Xu M, Li B (2018) Enhanced electrical conductivity of cellulose nanofiber/graphene composite paper with a sandwich structure. ACS Sustain Chem Eng 6:2983–2990. https://doi.org/10.1021/acssuschemeng.7b02683
Karimah A, Ridho MR, Munawar SS et al (2021) A review on natural fibers for development of eco-friendly bio-composite: characteristics, and utilizations. J Mater Res Technol 13:2442–2458
Krifa M (2021) Electrically conductive textile materials-application in flexible sensors and antennas. Textiles. https://doi.org/10.3390/textiles1020012
Li L, Ma Z, Xu P et al (2020) Flexible and alternant-layered cellulose nanofiber/graphene film with superior thermal conductivity and efficient electromagnetic interference shielding. Compos Part A Appl Sci Manuf 139:106134. https://doi.org/10.1016/J.COMPOSITESA.2020.106134
Liu X, Xiao W, Ma X et al (2020) Conductive regenerated cellulose film and its electronic devices–a review. Carbohydr Polym 250:116969. https://doi.org/10.1016/J.CARBPOL.2020.116969
López-Díaz D, Delgado-Notario JA, Clericò V et al (2020) Towards understanding the Raman spectrum of graphene oxide: the effect of the chemical composition. Coatings 10:524
Mittal H, Al Alili A, Morajkar PP, Alhassan SM (2021) GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose–a versatile adsorbent for the treatment of dyes contaminated wastewater. Int J Biol Macromol 167:1248–1261. https://doi.org/10.1016/J.IJBIOMAC.2020.11.079
Pan S, Wang P, Liu P et al (2021) Stable cellulose/graphene inks mediated by an inorganic base for the fabrication of conductive fibers. J Mater Chem C 9:5779–5788
Rafieian F, Hosseini M, Jonoobi M, Yu Q (2018) Development of hydrophobic nanocellulose-based aerogel via chemical vapor deposition for oil separation for water treatment. Cellulose 25:4695–4710
Raza MA, Rehman ZU, Ghauri FA (2018) Corrosion study of silane-functionalized graphene oxide coatings on copper. Thin Solid Films 663:93–99. https://doi.org/10.1016/J.TSF.2018.07.046
Salwa S, Nor M, Fazly M et al (2022) Surface modification of bio-based composites via silane treatment: a short review. J Adhesion Sci Technol. https://doi.org/10.1080/01694243.2022.2049087
Tan X, Peng Q, Yang K et al (2022) Preparation and Characterization of corn husk nanocellulose coating on electrospun polyamide 6. Alexandria Eng J 61:4529–4540. https://doi.org/10.1016/J.AEJ.2021.10.011
Valentini L, Cardinali M, Fortunati E et al (2013) A novel method to prepare conductive nanocrystalline cellulose/graphene oxide composite films. Mater Lett 105:4–7. https://doi.org/10.1016/J.MATLET.2013.04.034
Wilamowska-Zawlocka M, Puczkarski P, Grabowska Z et al (2016) Silicon oxycarbide ceramics as anodes for lithium ion batteries: influence of carbon content on lithium storage capacity. Rsc Adv 6:104597–104607
Wu Z, Li Y, Zhang L et al (2017) Thiol-ene click reaction on cellulose sponge and its application for oil/water separation. Rsc Adv. https://doi.org/10.1039/c7ra00847c
Yasin G, Arif M, Nizam MN et al (2018) Effect of surfactant concentration in electrolyte on the fabrication and properties of nickel-graphene nanocomposite coating synthesized by electrochemical co-deposition. RSC Adv 8:20039–20047
Zhang K, Zhou M, Cheng F et al (2022a) Preparation and characterization of starch-based nanocomposites reinforced by graphene oxide self-assembled on the surface of silane coupling agent modified cellulose nanocrystals. Int J Biol Macromol 198:187–193. https://doi.org/10.1016/J.IJBIOMAC.2021.12.136
Zhang Y, Wang W, Zhang F et al (2022b) Micro-diamond assisted bidirectional tuning of thermal conductivity in multifunctional graphene nanoplatelets/nanofibrillated cellulose films. Carbon N Y 189:265–275. https://doi.org/10.1016/J.CARBON.2021.12.067
Acknowledgements
This work was supported by the project ‘Advanced structures for thermal insulation in extreme conditions’ (Reg. No. 21–32510 M) granted by the Czech Science Foundation (GACR) and was supported by the research project of Student Grant Competition of Technical University of Liberec no. 2022-6069 and no. 2020-6031 granted by Ministry of Education Youth and Sports of Czech Republic.
Funding
Open access publishing supported by the National Technical Library in Prague. This work was supported by the project ‘Advanced structures for thermal insulation in extreme conditions’ (Reg. No. 21–32510 M) granted by the Czech Science Foundation (GACR).
Author information
Authors and Affiliations
Contributions
Conceptualization, XT, JS and JW; data curation, XT; formal analysis, XT and WX; resources. JS, JW, MV, AM, HL and JM; software, XT, QP and YJ; writing-original draft, XT, QP and WX; writing-review and editing, XT, AM, HL, PK, JM and WX; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
Manuscripts reporting studies that do not involve human participants, human data, or human tissue.
Consent for publication
All co-authors agree to submit the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, X., Jiang, Y., Peng, Q. et al. Development and characterization of silane crosslinked cellulose/graphene oxide conductive hydrophobic membrane. Cellulose 30, 4561–4574 (2023). https://doi.org/10.1007/s10570-023-05079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05079-x