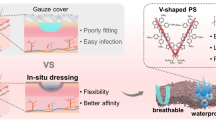
Abstract
Hydrogels, three-dimensional hydrophilic polymer cross-linked networks, have attracted much attention in the fields of biomedicine, health care, etc., due to their unique water retention, and structural similarity to extracellular matrix. However, the conventional synthetic polymer hydrogels have poor biocompatibility, adhesion, and biodegradability. Polysaccharides as a major constituent of native extracellular matrix are biocompatible and degradable, so the polysaccharides hydrogels are outstanding candidates for biomedicine and health care applications. Herein, novel hydrogel-based nanofiber membranes with adhesive, sustained-release, antibacterial and biocompatible performance were developed by complexing functionalized nanofibers membranes and polysaccharide hydrogels. The functionalized nanofiber membranes loaded with poly-lysine and allantoin were obtained by green solvent electrospinning technology using non-toxic and biological polycaprolactone as the base material. Biocompatible polysaccharide hydrogels were prepared by dynamic Schiff base crosslinking of chitosan oligosaccharide and oxidized konjac glucomannan. The dynamic Schiff base bonds endow hydrogels with outstanding self-healing properties. Consequently, with the integrated features of fibrous membranes and hydrogels, hydrogel-based nanofiber membranes have outstanding antibacterial capability against both Escherichia coli (99.99%) and Staphylococcus aureus (99.99%), sustained-release characteristic with 65.7% cumulative allantoin release during 10 h, and biocompatibility with at least 450% cell viability within 5 days. This unique composite structure exhibits positive potential in the application of skincare, including facial masks, medical dressings and other products.









Similar content being viewed by others
References
Bai JH, Wang R, Wang XM, Liu SD, Wang XL, Ma JM, Qin ZH, Jiao TF (2021a) Biomineral calcium-ion-mediated conductive hydrogels with high stretchability and self-adhesiveness for sensitive iontronic sensors. Cell Rep Phys Sci 2:11. https://doi.org/10.1016/j.xcrp.2021.100623
Bai JH, Wang R, Ju MX, Zhou JX, Zhang LX, Jiao TF (2021b) Facile preparation and high performance of wearable strain sensors based on ionically cross-linked composite hydrogels. Sci China Mater 64:942–952. https://doi.org/10.1007/s40843-020-1507-0
Choi YS, Hong SR, Lee YM, Song KW, Park MH, Nam YS (1999) Studies on gelatin-containing artificial skin: II. Preparation and characterization of cross-linked gelatin-hyaluronate sponge. J Biomed Mater Res 48:631–639. https://doi.org/10.1002/(SICI)1097-4636(1999)48:5%3c631::AID-JBM6%3e3.0.CO;2-Y
Chang SS, Lu WY, Park SH, Kang DH (2010) Control of foodborne pathogens on ready-to-eat roast beef slurry bys-polylysine. Int J Food Microbiol 141:236–241. https://doi.org/10.1016/j.ijfoodmicro.2010.05.021
Ce N, Norena CPZ, Brandelli A (2012) Antimicrobial activity of chitosan films containing nisin, peptide P34, and natamycin. CYTA-J Food 10:21–26. https://doi.org/10.1080/19476337.2010.537371
Chen HL, Cheng JW, Ran LX, Yu K, Lu BT, Lan GQ, Dai FY, Lu F (2018a) An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym 201:522–531. https://doi.org/10.1016/j.carbpol.2018.08.090
Chen T, Chen YJ, Rehman HU, Chen Z, Yang Z, Wang M, Li H, Liu HZ (2018b) Ultratough, self-Healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl Mater Interfaces 10:33523–33531. https://doi.org/10.1021/acsami.8b10064
Dong R, Zhao X, Guo B, Ma PX (2016) Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl Mater Interfaces 8:17138. https://doi.org/10.1021/acsami.6b04911
Fujioka K, Maeda M, Hojo T, Sano A (1998) Protein release from collagen matrices. Adv Drug Deliv Rev 31:247–266. https://doi.org/10.1016/S0169-409X(97)00119-1
Feng ZJ, Su Q, Zhang CN (2020) Bioinspired nanofibrous glycopeptide hydrogel dressing for accelerating wound healing: a cytokine-free, M2-type macrophage polarization approach. Adv Funct Mater. https://doi.org/10.1002/adfm.202006454
Ghobril C, Grinstaff MW (2015) The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem Soc Rev 44:1820–1835. https://doi.org/10.1039/C4CS00332B
Hennink WE, Nostrum CFV (2012) Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 54:13–36. https://doi.org/10.1016/j.addr.2012.09.009
Huang YC, Chu HW, Huang CC, Wu WC, Tsai JS (2015) Alkali-treated konjac glucomannan film as a novel wound dressing. Carbohydr Polym 117:778–787. https://doi.org/10.1016/j.carbpol.2014.10.047
Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29:322–337. https://doi.org/10.1016/j.biotechadv.2011.01.005
Kopecek J (2007) Hydrogel biomaterials: a smart future? Biomaterials 28:5185–5192. https://doi.org/10.1016/j.biomaterials.2007.07.044
Korkiatithaweechai S, Umsarika P, Praphairaksit N, Muangsin N (2011) Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar Drugs 9:1649–1663. https://doi.org/10.3390/md9091649
Li L, Wang N, Jin X, Deng R, Nie SH, Sun L, Wu QJ, Wei YQ, Gong CY (2014) Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 35:3903–3917. https://doi.org/10.1016/j.biomaterials.2014.01.050
Liang D, Lu Z, Yang H, Gao J, Chen R (2016) A novel asymmetric wettable AgNPs/chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl Mater Interfaces 8:3958–3968. https://doi.org/10.1021/acsami.5b11160
Liu HC, Sui XF, Xu H, Zhang LP, Zhong Y, Mao ZP (2016) Self-healing polysaccharide hydrogel based on dynamic covalent enamine bonds. Macromol Mater Eng 6:725–732. https://doi.org/10.1002/mame.201600042
Lu Z, Gao JT, He QF, Wu J, Liang DH, Yang H, Chen R (2017) Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr Polym 156:460–469. https://doi.org/10.1016/j.carbpol.2016.09.051
Mbosso EJT, Ngouela S, Nguedia JCA, Beng VP, Rohmer M, Tsamo E (2010) In vitro antimicrobial activity of extracts and compounds of some selected medicinal plants from Cameroon. J Ethnopharmacol 128:476–481. https://doi.org/10.1016/j.jep.2010.01.017
Popat A, Liu J, Lu GQ, Qiao SZ (2012) A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J Mater Chem 22:11173–11178. https://doi.org/10.1039/C2JM30501A
Suphat K, Pornpusadee U, Narong P, Nongnuj M (2011) Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar Drugs 9:1649–1663. https://doi.org/10.3390/md9091649
Shahbuddin M, Bullock AJ, Macneil S, Rimmer S (2014) Glucomannan-poly(N-vinyl pyrrolidinone) bicomponent hydrogels for wound healing. J Mater Chem B 2:727–738. https://doi.org/10.1039/c3tb21640c
Song JW, Yuan CQ, Jiao TF, Xing RR, Yang MY, Adams DJ, Yan XH (2020) Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small 16:8. https://doi.org/10.1002/smll.201907309
Taylor DL, Panhuis MHI (2016) Self-healing hydrogels. Adv Mater 28:9060–9093. https://doi.org/10.1002/adma.201601613
Vo DT, Sabrina S, Lee CK (2017) Silver deposited carboxymethyl chitosan-grafted magnetic nanoparticles as dual action deliverable antimicrobial materials. Mater Sci Eng C Mater Biol Appl 73:544–551. https://doi.org/10.1016/j.msec.2016.12.066
Wen X, Cao X, Yin Z, Wang T, Zhao C (2009) Preparation and characterization of konjac glucomannan–poly(acrylic acid) IPN hydrogels for controlled release. Carbohydr Polym 78:193–198. https://doi.org/10.1016/j.carbpol.2009.04.001
Wu TF, Du YC, Yan N, Farnood R (2015) Cellulose fiber networks reinforced with glutaraldehyde-chitosan complexes. J Appl Polym Sci. https://doi.org/10.1002/app.42375
Wang T, Ren XY, Bai Y, Liu L, Wu GF (2020) Adhesive and tough hydrogels promoted by quaternary chitosan for strain sensor. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2020.117298
Xing RT, Liu K, Jiao TF, Zhang N, Ma K, Zhang RY, Zou QL, Ma GH, Yan XH (2016) An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv Mater 28:3669–3676. https://doi.org/10.1002/adma.201600284
Yu HQ, Lu J, Xiao CB (2007) Preparation and properties of novel hydrogels from oxidized konjac glucomannan cross-linked chitosan for in vitro drug delivery. Macromol Biosci 7:1100–1111. https://doi.org/10.1002/mabi.200700035
Yu HQ, Mao CB (2008) Synthesis and properties of novel hydrogels from oxidized konjac glucomannan crosslinked gelatin for in vitro drug delivery. Carbohydr Polym 72:479–489. https://doi.org/10.1016/j.carbpol.2007.09.023
Zhao MH, Huang Z, Wang SX, Zhang LB (2020) Ultrahigh efficient and selective adsorption of Au(III) from water by novel chitosan-coated MoS2 biosorbents: performance and mechanisms. Chem Eng J 401:126006. https://doi.org/10.1016/j.cej.2020.126006
Acknowledgments
This work was partly supported by the Fundamental Research Funds for the Central Universities (2232020D-15, 2232020A-08, 2232020G-01, 2232020D-14 and 2232019D3-11) and Grants (51773037, 51973027, 51803023, 52003044 and 61771123) from the National Natural Science Foundation of China. This work has also been supported by the Chang Jiang Scholars Program and the Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-03-E00023) to Prof. Xiaohong Qin, the Shanghai Sailing Program (18YF 1400400), the Shanghai Sailing Program (19YF1400700), the Opening Project of State Key Laboratory of High Performance Ceramics and Superfine Microstructure (SKL201906SIC), Young Elite Scientists Sponsorship Program by CAST and DHU Distinguished Young Professor Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors contributed to the writing of the manuscript and approved the final version. The author declares that there are no competing economic interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Gu, J., Hao, Y. et al. Adhesive, sustained-release, antibacterial, cytocompatible hydrogel-based nanofiber membrane assembled from polysaccharide hydrogels and functionalized nanofibers. Cellulose 30, 323–337 (2023). https://doi.org/10.1007/s10570-022-04894-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04894-y