Abstract
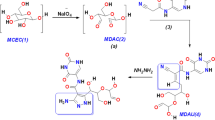
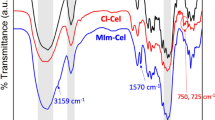
In this study, new amino heterocyclic cellulose derivatives were prepared. Dialdehyde cellulose was functionalized by Schiff base reaction with (E)-2-(4-(dimethylamino) benzylidene)-4-oxo-4-phenylbutanehydrazide, (E)-2-((1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-4-phenylbutane hydrazide, and thiophene-2-carbohydrazide. The prepared derivatives were characterized and confirmed by Fourier-transform infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray, and Thermo gravimetric analysis. Additionally, antimicrobial activity of all derivatives was assessed as well as antitumor activity. Results revealed that, all derivatives have potential antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Candida albicans, Cryptococcus neoformance, Aspergillus niger, A. fumigatus. Additionally, (E)-2-(4-(dimethylamino) benzylidene)-4-oxo-4-phenylbutanehydrazide and (E)-2-((1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-4-phenylbutanehydrazide cellulose compounds have good antitumor activities against Hep G2 and MCF7 cancerous cell lines without any effects on Wi38 normal cell line. Molecular dynamics study revealed that (E)-2-(4-(dimethylamino) benzylidene)-4-oxo-4-phenylbutanehydrazide and (E)-2-((1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-4-phenylbutanehydrazide cellulose derivatives have selectively target the ATP binding pocket residues. Identification of these ATP binding site residues and their crucial roles could provide the structure basis for understanding c-Kit kinase auto-inhibition











Similar content being viewed by others
References
Abdelraof M, Hasanin MS, El-Saied H (2019a) Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr Polym 211:75–83
Abdelraof M, Hasanin MS, Farag MM, Ahmed HY (2019b) Green synthesis of bacterial cellulose/bioactive glass nanocomposites: Effect of glass nanoparticles on cellulose yield, biocompatibility and antimicrobial activity. Int J Biol Macromole 138:975–985
Abdelraof M, Ibrahim S, Selim MA, Hasanin M (2020) Immobilization of L-methionine γ-lyase on different cellulosicmaterialsand its potential application in green-selective synthesis of volatile sulfur compounds. J Environ Chem Eng 8:103870
Abou-Elmagd WS, EL-Ziaty AK, Elzahar MI, Ramadan SK, Hashem AI (2016) Synthesis and antitumor activity evaluation of some N-heterocycles derived from pyrazolyl-substituted 2 (3 H)-furanone. Synth Commun 46:1197–1208
Abu-Elghait M, Hasanin M, Hashem AH, Salem SS (2021) Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: characterization, antibiofilm and biocompatibility. Int J Biol Macromole 175:294–303. https://doi.org/10.1016/j.ijbiomac.2021.02.040
Ali I, Mukhtar SD, Hsieh MF, Alothman ZA, Alwarthan A (2018) Facile synthesis of indole heterocyclic compounds based micellar nano anti-cancer drugs. RSC Adv 8:37905–37914
Berendsen HJ, Postma Jv, van Gunsteren WF, DiNola A, Haak J (1984) Molecular dynamics with coupling to an external bath. The Journal of chemical physics 81:3684-3690
Ciechanska D (2004) Multifunctional bacterial cellulose/chitosan composite materials for medical applications fibres. Text East Eur 12:69–72
Cournia Z, Allen B, Sherman W (2017) Relative binding free energy calculations in drug discovery: recent advances and practical considerations. J Chem Inf Modeling. 57:2911-2937 https://doi.org/10.1021/acs.jcim.7b00564
Dacrory S, Haggag ESA, Masoud AM, Abdo SM, Eliwa AA, Kamel S (2020) Innovative synthesis of modified cellulose derivative as a uranium adsorbent from carbonate solutions of radioactive deposits. Cellulose 27:7093–7108. https://doi.org/10.1007/s10570-020-03272-w
Dacrory S, Hashem AH, Hasanin M (2021) Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility Environmental Nanotechnology. Monitoring & Management 15:100453. https://doi.org/10.1016/j.enmm.2021.100453
de Coaña YP, Choudhury A, Kiessling R (2015) Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med 21:482–491
Desai N, Pandya D, Vaja D (2018) Synthesis and antimicrobial activity of some heterocyclic compounds bearing benzimidazole and pyrazoline motifs. Med Chem Res 27:52–60
Drissi M, Benhalima N, Megrouss Y, Rachida R, Chouaih A, Hamzaoui F (2015) Theoretical and experimental electrostatic potential around the m-nitrophenol molecule. Molecules 20:4042–4054. https://doi.org/10.3390/molecules20034042
El-Naggar ME, Hasanin M, Youssef AM, Aldalbahi A, Newehy M, Abdelhameed RM (2020) Hydroxyethyl cellulose/bacterial cellulose cryogel dopped silver@ titanium oxide nanoparticles: antimicrobial activity and controlled release of Tebuconazole fungicide. Int J Biol Macromole. https://doi.org/10.1016/j.ijbiomac.2020.09.226
EL-Sayed NS, El-Ziaty A, El-Meligy MG, Nagieb ZA (2017) Syntheses of New Antimicrobial Cellulose Materials Based 2-((2-aminoethyl) amino)-4-aryl-6-indolylnicotinonitriles. Egyptian J Chem 60: 465–477
Elbahnasawy MA, Shehabeldine AM, Khattab AM, Amin BH, Hashem AH (2021) Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: characterization and anticandidal activity. J Drug Delivery Sci Technol 62:102401
Felício MR, Silva ON, Gonçalves S, Santos NC, Franco OL (2017) Peptides with dual antimicrobial and anticancer activities. Front Chem 5:5
Fouda A, Khalil A, El-Sheikh H, Abdel-Rhaman E, Hashem A (2015) Biodegradation and detoxification of bisphenol-A by filamentous fungi screened from nature. J Adv Biol Biotechnol 2:123–132
Genheden S, Ryde U (2015a) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449–461
Genheden S, Ryde U (2015b) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. J Expert Opinion on Drug Discovery 10:449–461
Han S, Lee M, Kim BK (2010) Crosslinking reactions of oxidized cellulose fiber I reactions between dialdehyde cellulose and multifunctional amines on lyocell fabric. J Appl Polymer Sci 117:682–690
Hasanin M, El-Henawy A, Eisa WH, El-Saied H, Sameeh M (2019) Nano-amino acid cellulose derivatives: eco-synthesis, characterization, and antimicrobial properties. Int J Biol Macromole 132:963–969
Hasanin MS, Moustafa GO (2020) New potential green, bioactive and antimicrobial nanocomposites based on cellulose and amino acid International. J Biol Macromole 144:441–448
Hashem AH, Abdelaziz AM, Askar AA, Fouda HM, Khalil AMA, Abd-Elsalam KA, Khaleil MM (2021a) Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against rhizoctonia solani in faba bean plants. J Fungi 7:195
Hashem AH, Hasanin MS, Khalil AMA, Suleiman WB (2019) Eco-green conversion of watermelon peels to single cell oils using a unique oleaginous fungus: lichtheimia corymbifera AH13. Waste and Biomass Valorization 1:5721
Hashem AH, Khalil AMA, Reyad AM, Salem SS (2021b) Biomedical applications of mycosynthesized selenium nanoparticles using penicillium expansum ATTC 36200. Biol Trace Element Res. https://doi.org/10.1007/s12011-020-02506-z
Hashem AH, Saied E, Hasanin MS (2020a) Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustainable Chem Pharm 18:100333
Hashem AH, Suleiman WB, Abu-elreesh G, Shehabeldine AM, Khalil AMA (2020b) Sustainable lipid production from oleaginous fungus syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresource Technol Reports 12:100569. https://doi.org/10.1016/j.biteb.2020.100569
Hashem AI, Abou-Elmagd WS, El-Ziaty AK, Ramadan SK (2017) Ring Transformation of a 2 (3H)-furanone derivative into oxazinone and pyrimidinone heterocycles. J Heterocyclic Chem 54:3711–3715
Hayes JM, Archontis G (2012a) MM-GB (PB) SA calculations of protein-ligand binding free energies. In: Molecular Dynamics-Studies of Synthetic Biological Macromolecules
Hayes JM, Archontis G (2012b) MM-GB (PB) SA calculations of protein-ligand binding free energies. In: Molecular Dynamics-Studies of Synthetic and Biological Macromolecules. IntechOpen,
Hoenich NA (2006) Cellulose for medical applications: past, present, and future. BioResources 1:270–280
Hospital A, Goñi JR, Orozco M, Gelpí JL (2015) Molecular dynamics simulations: advances and applications. J Advances applications in bioinformatics chemistry: AABC 8:37
Hou T, Wang J, Li Y, Wang W (2010) Assessing the performance of the MM/PBSA and MM/GBSA methods. 1 the accuracy of binding free energy calculations based on molecular dynamics simulations. J Chem Inf Model 51:69–82
Ioset J-R, Brun R, Wenzler T, Kaiser M, Yardley V (2009) Drug Screening for Kinetoplastids Diseases A Training Manual for Screening in Neglected Diseases
Ismail MF, El-sayed AA (2019) Synthesis and in-vitro antioxidant and antitumor evaluation of novel pyrazole-based heterocycles. J Iran Chem Soc 16:921–937
Kalmoush A, El-Sakhawy M, Kamel S, Salama A, Hesemann P (2020) A green method for preparation of amino acids functionalized 2, 3-dialdehyde cellulose Egyptian. J Chem 63:8–9
Kelloff GJ (1999) Perspectives on cancer chemoprevention research and drug development. Advances in cancer research. Elsevier, London, pp 199–334
Khalil A, Abdelaziz A, Khaleil M, Hashem A (2020) Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett Appl Microbiol 72:263–274
Khalil AMA, Hashem AH (2018) Morphological changes of conidiogenesis in two aspergillus species. J Pure Appl Microbiol 12:2041–2048
Khan R, Rastogi R (1991) A convenient and facile synthesis of 2-arylidene-4-phenylbut-3-en-4-olides by use of N, N-dimethyl (chlorosulphonyl) methaniminium chloride as a cyclodehydrating agent. J Chem Res Synopses (Print)
Kim U-J, Lee YR, Kang TH, Choi JW, Kimura S, Wada M (2017) Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohyd Polym 163:34–42
Lee T-S et al (2018) GPU-accelerated molecular dynamics and free energy methods in Amber: performance enhancements and new features. J Chem Inf 58:2043–2050
Leguy J, Nishiyama Y, Jean B, Heux L (2018) Ultrastructural characterization of the core-shell structure of a wide range of periodate-oxidized cellulose from different native sources by solid-state 13C CP-MAS NMR. ACS Sustain Chem Eng 7:412–420
Li M-H, Luo Q, Xue X-G, Li Z-S (2011) Molecular dynamics studies of the 3D structure and planar ligand binding of a quadruplex dimer. J Mole Model. https://doi.org/10.1007/s00894-010-0746-0
Lindh J, Carlsson DO, Strømme M, Mihranyan A (2014) Convenient one-pot formation of 2,3-dialdehyde cellulose beads via periodate oxidation of cellulose in water. Biomacromol 15:1928–1932. https://doi.org/10.1021/bm5002944
Lucia A, van Herwijnen HW, Oberlerchner JT, Rosenau T, Beaumont M (2019) Resource-saving production of dialdehyde cellulose: optimization of the process at high pulp consistency. Chemsuschem 12:4679–4684
Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, Baptista PV, Fernandes AR (2015) Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 20:16852–16891
Mol CD et al (2004) Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem 279:31655–31663
Mustafa YF (2018) Synthesis, characterization and antibacterial activity of novel heterocycle, coumacine, and two of its derivatives Saudi. Pharm J 26:870–875
Muthukumar T, Sambandam B, Aravinthan A, Sastry TP, Kim J-H (2016) Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem 51:384–391
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Reflections on chemdraw. Chem Eng News Archive. https://doi.org/10.1021/cen-09233-scitech1
Reid JR, Heindel ND (1976) Improved syntheses of 5-substituted-4-amino-3-mercapto-(4H)-1, 2, 4-triazoles. J Heterocyclic Chem 13:925–926
Roe DR, Cheatham TE III (2013) PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput 9:3084–3095
Seifert E (2014) OriginPro 9.1: scientific data analysis and graphing software software review. ACS Publications
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015 CA: a cancer. J Clin 65:5
Sitkoff D, Sharp KA, Honig B (1994) Accurate calculation of hydration free energies using macroscopic solvent models. J Phys Chem 98:1978–1988
Standards NCfCL (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts. National Committee for Clinical Laboratory Standards Wayne, PA,
Suleiman W, El-Sheikh H, Abu-Elreesh G, Hashem A (2018a) Recruitment of cunninghamella echinulata as an Egyptian isolate to produce unsaturated fatty acids. Res J Pharm Biol Chem Sci 9:764–774
Suleiman W, El-Skeikh H, Abu-Elreesh G, Hashem A (2018b) Isolation and screening of promising oleaginous Rhizopus Sp and designing of taguchi method for increasing lipid production. J Innov Pharma Biol Sci 5:8–15
Sung B, Prasad S, Yadav VR, Aggarwal BB (2012) Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr Cancer 64:173–197
Valgas C, Souza SMD, Smânia E, Smânia A (2007) Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 38:369–380
Van de Loosdrecht A, Beelen R, Ossenkoppele g, Broekhoven M, Langenhuijsen M (1994) A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174: 311-320
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mole Graphics Modell 25:247–260
Webb B, Sali A (2014) Protein structure modeling with modeller. Protein Structure Prediction. Springer, NewYork, pp 1–15
Yusefi M et al (2020) The potential anticancer activity of 5-fluorouracil loaded in cellulose fibers isolated from rice straw. Int J Nanomed 15:5417
Acknowledgments
The authors would like to acknowledge the support for this research from National Research Centre, Cairo, Egypt; and Faculty of Science, Al-Azhar University, Cairo, Egypt.
Funding
All expenses whether chemical analysis or molecular identification etc. were funded by ourselves and there is no funder or funding agency support us to finish this work.
Author information
Authors and Affiliations
Contributions
MH research conceptualization, data curation, investigation, methodology, software, writing review and editing; AHH research conceptualization, data curation, investigation, methodology, software, writing review and editing; AAEl-R data curation, investigation, methodology, software, writing review and editing; SK research conceptualization, data curation, investigation, methodology, software, writing review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasanin, M., Hashem, A.H., El-Rashedy, A.A. et al. Synthesis of novel heterocyclic compounds based on dialdehyde cellulose: characterization, antimicrobial, antitumor activity, molecular dynamics simulation and target identification. Cellulose 28, 8355–8374 (2021). https://doi.org/10.1007/s10570-021-04063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04063-7