Abstract
In this work, deep eutectic solvent (DES) based on imidazole and triethylmethylammonium chloride was used as a reaction medium for the esterification of cellulose nanofiber (CNF) and all-cellulose composite (ACC) films with n-octylsuccinic anhydride (OSA) to obtain high strength and sustainable films with increased hydrophobicity. Diffuse reflectance infrared Fourier transform spectroscopy and X-ray photoelectron spectroscopy were used to prove the success of the modification. The mechanical strength of the modified films was analyzed in dry, humid, and wet conditions, and the hydrophobicity of the films was indicated in terms of contact angle measurements. In addition, water absorption and transparency of the films were characterized. The modification was proven to be simple and fast, and mild conditions of 80 °C reaction temperature and 1 h reaction time were used. DES/OSA- modified CNF film exhibited better mechanical properties in dry, humid, and wet conditions compared to reference CNF film, and DES/OSA-modified ACC film displayed notable higher mechanical properties in wet state compared to that of reference CNF film (31 MPa tensile strength and 6.1% strain at break vs. 18 MPa and 2.2%, respectively). These improvements were partly attributed to higher contact angles of modified films (ACC-DES/OSA 60° and CNF-DES/OSA 51°) compared to CNF film (37°).
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Earth’s human population is growing at an increasing pace, directly leading to an increase in the consumption of materials derived from fossil resources. This has been a driving force for researching and developing more sustainable, renewable, and biodegradable materials for replacing current petroleum-based products. One of those so-called green materials is cellulose, which is the most abundant biopolymer on Earth (Barnes et al. 2009; Klemm et al. 2011; Lee, 2014). In particular, its nano-sized form, i.e., nanocellulose, which includes cellulose nanofibers (CNF) and cellulose nanocrystals (CNC), has received great interest due to its unique properties. For example, CNF has a special ability to form strong films because of its inherent tendency to create entangled and hydrogen-bonded networks (Nair et al. 2014).
Films derived from CNF have very good mechanical properties. It is reported that CNF films can have a modulus above 16 GPa and a tensile strength above 250 MPa (Henriksson et al. 2008; Sehaqui et al. 2010; Yang et al. 2020; Sirviö et al. 2014; Liimatainen et al. 2013). CNF films also have a good resistance for grease as well as excellent oxygen barrier properties in dry conditions. For these reasons, CNF films have been introduced as green alternatives in many applications such as food packaging (Nair et al. 2014; Aulin et al. 2010; Wang et al. 2018), organic light-emitting diode (OLED) displays (Zhu et al. 2014), and organic solar cells (Fang et al. 2014).
All-cellulose composite (ACC) is a special type of biocomposite where both the matrix and reinforcement phase are based on cellulose. ACCs are green and recyclable, and chemical similarity of components promotes good fiber–matrix adhesion (Nishino et al. 2004; Kalka et al. 2014). ACCs are produced through two distinct strategies. Dissolved cellulose can simply be mixed with reinforcing cellulose fibers followed by casting and regeneration. Another approach is to partially dissolve the surface of the cellulose fiber network and then regenerate in situ to form a continuous matrix around the fibers (Huber et al. 2012). Several different solvent systems have been studied for partial dissolution, including dimethylacetamide/LiCl (Nishino et al. 2004), N-methylmorpholine N-oxide (NMMO) (Ouajai and Shanks, 2009), ionic liquids (Zhang et al. 2016), NaOH–urea (Piltonen et al. 2016), and tetraethylammonium hydroxide (TEAOH) (Sirviö et al. 2017). Among them, TEAOH has been reported to be simple and efficient (30 s at room temperature) for ACC preparation (Sirviö et al. 2017).
Despite the appealing properties of CNF films and ACCs, poor moisture and water tolerance limit their utilization in many applications. Water weakens the internal hydrogen bonds of cellulose and promotes interfibrillar slippage when introduced to external stress, resulting in decreased mechanical properties (Sehaqui et al. 2014; Benitez and Walther, 2017; Yousefi et al. 2013). Benitez et al. (2013) and Sehaqui et al. (2014) addressed this issue by studying the mechanical properties of CNF films in different relative humidities (RH). Both Young’s modulus and tensile strength were noted to decrease over 90% at 100% RH and wet conditions compared to dry conditions (0% RH).
Esterification (Rodionova et al. 2011; Sehaqui et al. 2014) and silylation (Johansson et al. 2011; Peresin et al. 2017) have been widely studied to increase the hydrophobicity and improve the water resistance of cellulose structures. However, these chemical modifications require typically complicated or slow processing, and volatile organic solvents, such as dimethylacetamide (DMAc), are often harnessed (Sethi et al. 2018). In the present work, deep eutectic solvents (DESs) are used as a reaction media for hydrophobic modification of CNF and ACC films through esterification. DESs are typically generated by complexation of a quaternary ammonium or other cations with a hydrogen bond donor to form a eutectic mixture through self-association driven by hydrogen bond interactions. These solvents have a low toxicity, are biodegradable and readily available, and exhibit high thermal stability, relatively wide temperature range, and low vapor pressure (Zhang et al. 2012; Smith et al. 2014). DESs can act as solvents, reactants, and catalysts (Smith et al. 2014). For that reason, they are under intensive research in chemical synthesis (Liu et al. 2015), metal processing (Smith et al. 2014), and nanomaterial science (Abo-Hamad et al. 2015).
Recently, DESs have also been studied for pretreatment and modification of cellulose (Sirviö et al. 2015; Li et al. 2017; Selkälä et al. 2020). Sirviö et al. (2015) used DES of choline chloride–urea (molar ratio of 1:2) to promote nanofibrillation of birch pulp. Moreover, DES of imidazole and triethylmethylammonium chloride (TEMACl) was used as a reaction medium to produce anionic wood fibers from unbleached mechanical pulp, further processing it into wood nanofibers. The modification exhibited high yield (over 90%) using relatively mild reaction conditions (2 h at 70 °C) (Sirviö and Visanko, 2017).
This study introduces a novel, fast, and simple approach for surface modification of CNF and ACC films using DES of imidazole and TEMACl (molar ratio of 7:3) as a reaction media and n-octylsuccinic anhydride as a reagent. The modified CNF and ACC films were characterized using diffuse reflectance infrared Fourier transform spectroscopy (DRIFT), X-ray photoelectron spectroscopy (XPS), field emission scanning electron microscopy (FESEM), and ultraviolet–visible (UV–Vis) spectrometry. Mechanical properties of the films samples were investigated in different RH as well as at wet state. In addition, contact angle and water absorption measurements were performed for the film samples.
Materials and methods
Materials
Non-derivatized, pre-ground (Masuko super mass colloider MKCA6-2, Japan) dissolving pulp from softwood in aqueous solution (consistency of 1.66 wt.%) was used as a cellulose raw material for nanocellulose film production. Selkälä et al. 2018 describe in detail the grinding procedure. TEAOH (35 wt.% in H2O) was from Sigma-Aldrich. Imidazole (purity > 98.0%), TEMACl (purity > 98.0%), and n-octylsuccinic anhydride (OSA) (purity > 98.0%) were from Tokyo Chemical Industry Co. Ethanol (purity > 96%) and acetone (purity 100.0%) were from VWR International. Deionized water was used throughout the study, if not mentioned otherwise.
Nanofibrillation of cellulose
The pre-ground dissolving pulp was diluted to a 0.5 wt.% consistency and was then mixed at 10,000 rpm with an Ultra-Turrax mixer (IKA T25, Germany) for 1 min to achieve a homogeneous suspension. The suspension was nanofibrillated using a microfluidizer (Microfluidics M-110EH-30, USA). The sample passed the equipment for five times: once through 400-μm and 200-μm chambers at pressure of 1000 bar and four times through 400-μm and 100-μm chambers at pressure of 1500 bar. After nanofibrillation, samples were taken from the suspension and dried overnight at 100 °C to determine the dry matter content.
Preparation of cellulose nanofiber films
CNF films were prepared by measuring 0.265 g of dry nanofibrillated cellulose to a decanting glass and then diluting it by water to total weight of 100 g (0.265 wt.%). After this, the sample was degassed by mild ultrasonic treatment (Elmasonic P, Elma Schmidbauer GmbH, Germany) for 10 min. The sample was then vacuum filtrated on top of the membrane (Durapore DVPP 0.65 μm, Merck Millipore Ltd., Ireland) using negative pressure of approximately 800 mbar. After the film was formed and the excess water was removed, it was further dried using a vacuum dryer (Karl Schröder KG, Germany) at 93 °C using negative pressure of 900 mbar for 10 min. Finally, the basis weight of the film (69.3 g/m2) was measured.
Preparation of all-cellulose composite films
ACC film was fabricated by partially dissolving and regenerating of the prefabricated nanocellulose film in TEAOH solution for 30 s (Sirviö et al. 2017) and then washing it with ethanol four times for approximately 5 min each time. The film was then dried using a vacuum drier as described in the preparation of CNF films.
Preparation of deep eutectic solvent
DES of imidazole and TEMACl (molar ratio of 7:3) was prepared by weighing the components into the decanting glass and heating the mixture in the oil bath at 80 °C. When approximately half of the liquid was formed, a magnetic stirrer was enabled and kept constantly mixing to speed up the formation of DES until a clear liquid was obtained.
Modification of the CNF and ACC films with OSA in the DES
Prior to modification, the film (CNF or ACC) was washed with acetone to remove possible impurities that could disturb the reaction between the OSA and the cellulose film (Johansson et al. 2011). The reagent (OSA) was added into the DES, followed by submerging the film into the DES/OSA reagent system horizontally. A custom-made film protection design was used to prevent the magnetic stirrer from touching the film. The ratio between the reagent (OSA) and the film was 10:1 in weight, and the amount of DES (140 g) was selected so that there was enough liquid to cover the film fully. The reaction temperature was set to 80 °C. After 1 h reaction time, the film was washed three times (~ 5 min each time) with water to remove the DES and the reagent from the film. Fresh washing liquid was used in every washing step. Finally, the film was dried using a vacuum dryer for 10 min (Karl Schröder KG, Germany). Both CNF and ACC films were also treated in pure DES without any reagent (OSA). Table 1 summarizes the prepared film samples.
Characterization of film samples
Mechanical properties of the films were measured by a universal testing machine (Zwick Roell, Ulm, Germany) with a 2 kN load cell. The samples were prepared by cutting five strips from each film that is 5 mm wide and 50–70 mm long. The test was performed in 0%, 50%, and 100% relative humidity and at wet state. The films were kept in constant temperature (23 °C ± 1 °C) and humidity 48 h before analysis. For 0% humidity, the samples were dried and kept in a desiccator to absorb water in the silica gel. For 50% humidity, the samples were stored in a separate room with controlled conditions (50% ± 2.5% RH). For 100% humidity, the samples were kept in a desiccator, in which water absorbing silica gel was replaced with water. The analysis of fully wetted films was performed following the procedure reported by Sehaqui et al. (2014). In short, a drop of water was carefully placed on the middle of the strip and was let to spread approximately 2 cm in length for 60 s. The thickness of the strips was measured as an average of three random measuring points using Precision Thickness Gauge (FT3, Hanatek Instruments, UK). For tensile testing, the gauge length was set to 40 mm, and the strain was controlled at 5 mm/min with pre-strain value of 0.1 MPa and 1 MPa for the wet films. Young’s modulus was calculated from the slope of the line from the linear region of the stress–strain curve, and ultimate tensile strength was determined as the stress of specimen fracture.
Water absorption measurements were performed by weighing small specimen of the films in 0%, 50%, and 100% relative humidity and recording the average value of three separate specimens of each film. The samples were conditioned using the same procedure as in the mechanical tests and were kept in constant temperature (23 °C ± 1 °C) and humidity at least 48 h before the measurements.
The chemical characteristics of the films were analyzed using diffuse reflectance infrared Fourier transform spectroscopy (DRIFT). The spectra were recorded from small film specimens using Bruker Vertex 80v (USA). The wavelength range of 400–4000 cm−1 and total of 40 scans at a resolution of 4 cm−1 were used.
The films were imaged with a FESEM (Zeiss Sigma Ultra plus, Germany) at an accelerating voltage of 5.0 kV in order to analyze the surface and the cross section of the films. The samples were sputtered with platinum (High Resolution Sputter Coater, Agar Scientific, UK) before analysis using sputtering time of 30 s and current of 40 mA.
XPS was used to analyze the surface chemical composition of the films. XPS measurements were conducted with a Thermo Fisher Scientific ESCALAB 250Xi XPS system (USA) equipped with an Ar+ cluster ion gun with a total photoelectron energy resolution of 0.4 eV. The measurement area was less than 1 mm2, and the measurement depth less than 10 nm. Data analysis was performed using Avantage Software (UK). Atomic concentration ratios were calculated from the peak areas. The C 1 s, O 1 s, N 1 s, F 1 s, and Si 2p peaks were decomposed and analyzed and fitted with the software’s 70:30 Gaussian–Lorentzian product function.
The contact angle measurements were conducted using Krüss DSA100 (Germany) system. The system utilized high-speed camera (1000 fps) and drop analyzing software. During the measurements, the system recorded videos of the droplet behavior with various films. To avoid gravitational flattening, the droplet size of Milli-Q water was maintained at approximately 1 µl. Consequently, the initial contact angle information was extracted from the videos and depicted in Fig. 1. This step was followed by a time domain analysis of the droplet behavior on the film surface. In both cases, the contact angles were extracted by the height–width method; a rectangle enclosed by contour line is regarded as being the segment of a circle. As a result, the contact angle was calculated from the height–width relationship of the enclosing rectangle. For each sample, four droplets on different locations were analyzed, the obtained results were averaged, and standard deviations were calculated.
The optical transmittance of the films was measured on the wavelength of 200–800 nm using a UV–Vis spectrometer (Shimadzu, Japan). To assure that the films were perpendicularly aligned against the incoming beam and to avoid wrinkling, the films were put between two quartz glass slides before being set up in a cuvette stand. The absorption coefficient was calculated with the Beer–Lambert law:
where the transmittance (ratio of transmitted [I] and incoming [I0] flux) depends on the absorption coefficient μ and the path length of the light x.
Results and discussion
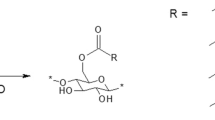
The self-standing CNF and ACC films were modified with n-octylsuccinic anhydride (OSA) using a DES of imidazole and TEMACl as a reaction medium. The reaction between hydroxyl groups of cellulose and OSA results in ring opening of anhydride group and attachment of a long alkyl chain on cellulose through esterification reaction, as shown in Fig. 2. DRIFT was used to analyze the chemical structure of the films in terms of ester bond formation (Fig. 3). The modified films, CNF-DES/OSA and ACC-DES/OSA, clearly possessed a new peak at wavenumber of 1750 cm−1 compared to reference samples without DES and/or OSA (CNF, CNF-DES, ACC, and ACC-DES), indicating the presence of C=O bond of the ester. Thus, the appearance of carbonyl peak in OSA-modified films indicated the successful esterification of CNF and ACC in the DES medium (Silverstein et al. 1981).
XPS analysis was used to characterize the surface chemical composition of the films and support the findings from the DRIFT spectra. Figure 4 presents the XPS carbon spectra of the films, and Table 2 presents the elemental and carbon bond concentration (Figures S1, S2, and S3 present the spectra of CNF-DES, ACC-DES, and the corresponding XPS survey spectra, respectively, which are found in Supplementary Information). The original CNF film had a C–C bond concentration of 8.7%, while the modified CNF film, CNF-DES/OSA, had a C–C bond concentration of 13.2%. This result indicated that the modification attached a long alkyl chain in the cellulose and increased the presence of C–C bonds in the cellulose structure. A similar observation was also observed for the ACC films, i.e., ACC-DES/OSA had a higher C–C bond concentration (10.1%) compared to that of ACC (8.2%). Both modified films, CNF-DES/OSA and ACC-DES/OSA, also had a higher O–C=O bond concentrations attributed to carbonyls (1.55% and 1.98%, respectively) compared to that of the original CNF film (0.97%), supporting the DRIFT findings of successful esterification reaction between cellulose and the OSA. Although pure cellulose does not contain peaks corresponding to.
O–C=O bond, these bonds can be formed during the pulping and bleaching processes, or the bonds are from hemicelluloses of the pulp (Bayer et al. 2016).
XPS was also used to investigate the possible traces of the DES in the films and indicate if the DES reacted with the films. In particular, the concentration of nitrogen was used to reveal the presence of DES in the samples. All films exhibited the N 1 s peak, but the amounts of nitrogen were small, ranging from 0.12% to 0.31% (Table 2). Thus, the modification increased the nitrogen concentrations only to some extent. For the CNF films, the amount of nitrogen increased from 0.12% (CNF) to 0.25% (CNF-DES/OSA); for the ACC films, the amount of nitrogen increased from 0.24% (ACC) to 0.31% (ACC-DES/OSA). This increase was presumably attributed to imidazole, which binds to carboxyls of the esterified films via electrostatic interactions. When OSA is used, imidazole is acting as base by neutralize the formed carboxylic acid and remains in the film as imidazolium-cation (see Fig. 1). Overall, ACC films had a higher concentration of nitrogen compared to CNF films. It is likely that TEAOH, which was used to prepare the ACC films and which contained nitrogen, existed also as traces in the ACC films. On the other hand, the differences in nitrogen concentrations are small and the residual content of TEAOH was not directly analyzed. The DES treatment without OSA did not alter the nitrogen content of the films (CNF-DES and ACC-DES) and showed that the DES did not react during the modification.
All of the samples contained small amounts of fluorine, which may originate from the polyvinylidene fluoride filter membrane used in the preparation of CNF films. Previously F 1 s peak of the nanocellulose films was proposed to be associated with the Teflon sheets used in the hot pressing of the films (Bayer et al. 2016). All the films also demonstrated a small Si 2 s peak, which can be from the grinding stones of the super mass colloider (Gane et al. 2010).
The influence of the OSA/DES modification on morphology and structure of the films was depicted with FESEM. Figure 5 illustrates the surface (a–d) and cross-sectional FESEM images (e–h) of the modified films (CNF-DES/OSA and ACC-DES/OSA) and the original film samples without OSA/DES treatments (CNF and ACC). Supplementary Information (Figures S4–S7) shows the samples from DES treatment without OSA (CNF-DES and ACC-DES). Overall, the DES medium or esterification with OSA only had a minor effect on the visual appearance of the films, and both original, DES-treated, and OSA/DES-modified films presented a similar structure both in surface and cross section. However, the surface pattern of the CNF and ACC films was different, and partial dissolution of the outermost surface structure of ACC by TEAOH treatment was clearly visible. The cross-sectional images (Fig. 5e–h) also illustrated that all films have a layered structure inside and suggested that the modification in DES and the dissolution of cellulose in TEAOH did not affect the internal morphology of the films.
Figure 6 presents the stress–strain curves of the films at different humidity, 0% RH, 50% RH, 100% RH, and wet state. At dry conditions (0% RH and 50% RH), the OSA-modified CNF film (CNF-DES/OSA) had the highest tensile strength (i.e., 211 MPa for dry CNF-DES/OSA film), while the original CNF film had the second highest tensile strength (i.e., 183 MPa for dry CNF film). Generally, all ACC films had a lower tensile strength and Young’s modulus than the CNF films (Table 3). This finding is likely attributed to the alteration of the crystal structure of cellulose in ACC films as it has previously been shown that the crystal structure of cellulose changes from cellulose I to cellulose II during the preparation of ACC (Sirviö et al. 2017). In turn, cellulose II has a lower modulus than cellulose I (Northolt et al. 2001). The ACC had a higher tensile strength and strain at break at 50% RH than the dry state (0% RH). ACC is brittle at dry conditions, since the regenerated matrix does not allow the composite to stretch as nanofibrils do, and the composite breaks. When RH is higher, water acts as a plasticizer for the matrix, whereupon the composite is able to stretch but as much as nanofibrils. When the composite is fully wetted, the matrix is plasticized, but the matrix promotes wet strength, and the slippage of the composite is not as severe as of the nanofibrils (Benitez et al. 2013; Sethi et al. 2019). For the other samples, tensile strengths and moduli are lower at 50% RH than at 0% RH, because water breaks the interfibrillar hydrogen bonds, causing interfibrillar slippage, thus allowing the film stretch under stress (Benitez and Walther, 2017). The curves are closer to each other, and transition from the linear elastic region to the strain-hardening region is more rapid.
At high humidity of 100% (Fig. 6c), tensile strengths and Young’s modulus of all films are decreased. The linear elastic region was shorter, while the strain-hardening region was longer compared to 50% RH. This behavior was noted as a higher strain at break of the samples. Overall, the ACC films had a lower tensile strength than CNF films at high RH similar to lower RH conditions.
As a whole, OSA/DES modification improved the mechanical performance of the films at humid conditions. The CNF-DES/OSA exhibited the highest stress at break at 0%, 50%, and 100% RH, and the tensile strength was also higher than that of the original CNF film at wet state. This behavior is likely attributed to the increased hydrophobicity of the films, which hindered the water interaction with the film matrix.
The wetting of films fully with water (instead of exposure to humidity) had a great influence on all the films, since tensile strengths and Young’s modulus dropped significantly in wet state (Fig. 6d). In addition, the stress–strain curves did not show a clear yield points at all, indicating that the films transferred straight into strain-hardening region and deformed (Benitez et al. 2013; Sethi et al. 2019). Compared with the humid conditions, the wet CNF and ACC films behaved differently, i.e., ACC films exhibited clearly higher tensile strengths (31–40 MPa) than that of CNF films (18–27 MPa). The highest tensile strength of 40.4 MPa and strain at break of 6.2% were measured with the ACC-DES film. In turn, the original CNF film had the lowest values (18.4 MPa and 2.2%, respectively). Moreover, OSA-modified films did not perform that well at wet state compared to the humid conditions. For example, ACC-DES/OSA film had the lowest tensile strength within ACC samples. The DES-treated films (without OSA) of both film types possessed the highest tensile strengths within their own film types, although the differences were not significant.
The influence of OSA/DES modification on the surface characteristics of the films was analyzed in terms of contact angle measurement. Esterification increased the contact angles of both films significantly. The contact angles of CNF-DES/OSA and ACC-DES/OSA were 51º and 60º, respectively, while the unmodified films, CNF and ACC, had contact angles of 37º and 36º, respectively. This result indicated that the modification increased the hydrophobicity of the film surfaces. However, the DES treatment without OSA had a different impact on contact angles of CNF and ACC films. ACC-DES had the lowest contact angle of all samples with contact angle of 25º, but CNF-DES has a contact angle of 44º. Figure 7 presents contact angles of the films as a function of time after exposing the film surface with water droplet. The contact angle of all the samples (except ACC) increased first slightly and reached its maximum value after 30–40 s. After that, the contact angle started to decline. It was noted that the films started to swell after 30–40 s. The measurement was difficult to perform for the ACC-DES sample because the drop could not hold together and it spread across the specimen. That effect caused the higher standard deviation.
As the OSA/DES modification increased the hydrophobicity of the films (as indicated by increased contact angles), moisture absorption measurements were done to investigate if the modification also affected the moisture uptake of the films. Figure 8 presents the cumulative moisture absorption of the films in different relative humidity ranges. At low humidity range (0–50% RH), the moisture uptake was small, being < 3.5% with all films. At high humidity (50–100% RH), the moisture uptake of all films increased remarkably, and ACC films absorbed more moisture than CNF films. Of the ACC films, the highest moisture uptake of 11% was observed with the unmodified film (ACC), while the DES treatment (ACC-DES) resulted in the lowest moisture uptake (9%). The OSA/DES treatment significantly decreased the moisture uptake of the CNF films. Generally, the unmodified films on both film types had the highest moisture uptake, and the modification of the films decreased the moisture uptake. Therefore, it is likely that the OSA modification decreased the number of hydrophilic sites on the surface of the film (Wang et al. 2018) as also suggested by the contact angle measurement. Earlier, the acetylation of nanocellulose films through esterification reaction has been reported to decrease the moisture uptake of the nanocellulose films (Wang et al. 2018).
During the preparation of the film samples, it was clearly observed that the optical properties of the films differed (Fig. 9). However, all of the films had a high transparency and transmittance of above 50% at visible light spectrum (> 371 nm), except the CNF film, which had a transmittance of above 50% only at higher wavelengths (> 493 nm) (Fig. 10). The DES treatment without OSA did not affect the transmittance of CNF films. All ACC films had a higher transmittance than the CNF films. For example, all ACC films had a transmittance of 75–80% at 600 nm. In addition to the differences in transmittance, the samples exhibited different optical haziness (see Fig. 9g–l). In particular, CNF films appeared hazier compared to the images of ACC films when the films were held ~ 2 cm above the background image. This difference might be attributed to differences in the (nano)particle dimensions and the porosity of the films (Zhu et al. 2014). In turn, FESEM surface images (Fig. 4a–d and Figure S4 and S5) illustrated that ACC films had a less porous and more uniform structure compared to CNF films, which decreased the light scattering in the ACC films.
Conclusions
DES of imidazole and TEMACl showed to be a novel, simple, and fast reaction medium for modifying CNF and ACC films. The films were successfully esterified with n-octylsuccinic anhydride in DES using relatively mild reaction conditions (1 h at 80ºC). The DES/OSA-modified CNF film exhibited better mechanical properties in 0%, 50%, and 100% RH compared to the reference CNF film. At wet state, ACC films had better mechanical properties than CNF films, although the DES/OSA-modified ACC film had slightly lower mechanical properties than the reference ACC film. The OSA modification increased the hydrophobicity of the film samples. Both DES/OSA-modified films showed higher contact angles than the reference film. In addition, DES/OSA-modified films had a lower moisture uptake compared to their reference films.
Data availability
All authors declare that all data and materials support their published claims and comply with field standards.
References
Abo-Hamad A, Hayyan M, AlSaadi MAH, Hashim MA (2015) Potential applications of deep eutectic solvents in nanotechnology. Chem Eng J 273:551–567. https://doi.org/10.1016/j.cej.2015.03.091
Aulin C, Gällsted M, Lindström T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574. https://doi.org/10.1007/s10570-009-9393-y
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Phil Trans R Soc B 364:1985–1998. https://doi.org/10.1098/rstb.2008.0205
Bayer T, Cunning BV, Selyanchyn R, Nishihara M, Fujikawa S, Sasaki K, Lyth SM (2016) High temperature proton conduction in nanocellulose membranes: paper fuel cells. Chem Mater 28:4805–4814. https://doi.org/10.1021/acs.chemmater.6b01990
Benitez AJ, Walther A (2017) Cellulose nanofibril nanopapers and bioinspired nanocomposites: a review to understand the mechanical property space. J Mater Chem A 5:16003–16024. https://doi.org/10.1039/c7ta02006f
Benitez AJ, Rendon-Torres J, Poutanen M, Walther A (2013) Humidity and multiscale structure govern mechanical properties and deformation modes in films of native cellulose nanofibrils. Biomacromol 14:4497–4506. https://doi.org/10.1021/bm401451m
Fang Z, Zhu H, Preston C, Hu L (2014) Development, application and commercialization of transparent paper. Transl Mater Res 1:015004. https://doi.org/10.1088/2053-1613/1/1/015004
Gane P, Schoelkopf J, Gantenbein D, Schenker M, Pohl M, Kubler B (2010) Process for the production of nano-fibrillar cellulose suspensions. WO 2010/112519 Al.
Henriksson M, Berglund LA, Isaksson P, Lindström T, Nishino T (2008) Cellulose nanopaper structures of high toughness. Biomacromol 9:1579–1585
Huber T, Müssig J, Curnow O, Pang S, Bickerton S, Staiger MP (2012) A critical review of all-cellulose composites. J Mater Sci 47:1171–1186. https://doi.org/10.1007/s10853-011-5774-3
Johansson LS, Tammelin T, Campbell JM, Setälä H, Österberg M (2011) Experimental evidence on medium driven cellulose surface adaptation demonstrated using nanofibrillated cellulose. Soft Matter 7:10917–10924. https://doi.org/10.1039/c1sm06073b
Kalka S, Huber T, Steinberg J, Baronian K, Müssig J, Staiger M (2014) Biodegradability of all-cellulose composite laminates. Compos Part A Appl Sci Manuf. https://doi.org/10.1016/j.compositesa.2013.12.012
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466. https://doi.org/10.1002/anie.201001273
Lee KY, Aitomäki Y, Berglund LA, Oksman K, Bismarck A (2014) On the use of nanocellulose as reinforcement in polymer matrix composites. Compos Sci Technol 105:15–27. https://doi.org/10.1016/j.compscitech.2014.08.032
Li P, Sirviö JA, Haapala A, Liimatainen H (2017) Cellulose Nanofibrils from nonderivatizing urea-based deep eutectic solvent pretreatments. ACS Appl Mater Interfaces 9:2846–2855. https://doi.org/10.1021/acsami.6b13625
Liimatainen H, Ezekiel N, Sliz R, Ohenoja K, Sirviö JA, Berglund L, Hormi O, Niinimäki J (2013) High-strength nanocellulose−talc hybrid barrier films. ACS Appl Mater Interfaces 5:13412–13418. https://doi.org/10.1021/am4043273
Liu P, Hao LP, Zhang ZH (2015) Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv 5:48675–48704. https://doi.org/10.1039/c5ra05746a
Nair SS, Zhu JY, Deng Y, Ragauskas AJ (2014) High performance green barriers based on nanocellulose. Sustainable Chem Processes 2:23. https://doi.org/10.1186/s40508-014-0023-0
Nishino T, Matsuda I, Hirao K (2004) All-cellulose composite. Macromolecules 37:7683–7687. https://doi.org/10.1021/ma049300h
Northolt MG, Boerstoel H, Maatman H, Huisman R, Veurik J, Elzerman H (2001) The structure and properties of cellulose fibers spun from an anisotropic phosphoric acid solution. Polymer 42:8249–8264
Ouajai S, Shanks RA (2009) Preparation, structure and mechanical properties of all-hemp cellulose biocomposites. Compos Sci Technol 69:2119–2126. https://doi.org/10.1016/j.compscitech.2009.05.005
Peresin MS, Kammiovirta K, Heikkinen H, Johansson LS, Vartiainen J, Setälä H, Österberg M, Tammelin T (2017) Understanding the mechanisms of oxygen diffusion through surface functionalized nanocellulose films. Carbohydr Polym 174:309–317. https://doi.org/10.1016/j.carbpol.2017.06.066
Piltonen P, Hildebrandt NC, Westerlind B, Valkama JP, Tervahartiala T, Illikainen M (2016) Green and efficient method for preparing all-cellulose composites with NaOH/urea solvent. Compos Sci Technol 135:153–158. https://doi.org/10.1016/j.compscitech.2016.09.022
Rodionova G, Lenes M, Eriksen Ø, Gregersen Ø (2011) Surface chemical modification of microfibrillated cellulose: improvement of barrier properties for packaging applications. Cellulose 18:127–134. https://doi.org/10.1007/s10570-010-9474-y
Sehaqui H, Liu A, Zhou Q, Berglund LA (2010) Fast preparation procedure for large, flat cellulose and cellulose/inorganic nanopaper structures. Biomacromol 11:2195–2198. https://doi.org/10.1021/bm100490s
Sehaqui H, Zimmerman T, Tingaut P (2014) Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 21:367–382
Selkälä T, Suopajärvi T, Sirviö JA, Luukkonen T, Lorite GS, Kalliola S, Sillanpää M, Liimatainen H (2018) Rapid uptake of pharmaceutical salbutamol from aqueous solutions with anionic cellulose nanofibrils: the importance of pH and colloidal stability in the interaction with ionizable pollutants. Chem Eng J 350:378–385. https://doi.org/10.1016/j.cej.2018.05.163
Selkälä T, Suopajärvi T, Sirviö JA, Luukkonen P, Braga de Carvalho ALC, Liimatainen H (2020) Surface modification of cured inorganic foams with cationic cellulose nanocrystals and their use as reactive filter media for anionic dye removal. ACS Appl Mater Interfaces 12:27745–27757. https://doi.org/10.1021/acsami.0c05927
Sethi J, Farooq M, Sain S, Sain M, Sirviö JA, Illikainen M, Oksman K (2018) Water resistant nanopapers prepared by lactic acid modified cellulose nanofibers. Cellulose 25:259–268. https://doi.org/10.1007/s10570-017-1540-2
Sethi J, Visanko M, Österberg M, Sirviö JA (2019) A fast method to prepare mechanically strong and water resistant lignocellulosic nanopapers. Carbohydr Polym 203:148–156. https://doi.org/10.1016/j.carbpol.2018.09.037
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrometric identification of organic compounds, 4th edn. John Wiley and Sons, New York
Sirviö JA, Visanko M (2017) Anionic wood nanofibers produced from unbleached mechanical pulp by highly efficient chemical modification. J Mater Chem A 5:21828–21835. https://doi.org/10.1039/c7ta05668k
Sirviö JA, Kolehmainen A, Visanko M, Liimatainen H, Niinimäki J, Hormi O (2014) Strong, self-standing oxygen barrier films from nanocelluloses modified with regioselective oxidative treatments. ACS Appl Mater Interfaces 6:14384–14390. https://doi.org/10.1021/am503659j
Sirviö JA, Visanko M, Liimatainen H (2015) Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem 17:3401–3406. https://doi.org/10.1039/c5gc00398a
Sirviö JA, Visanko M, Hildebrandt NC (2017) Rapid preparation of all-cellulose composites by solvent welding based on the use of aqueous solvent. Eur Polym J 97:292–298. https://doi.org/10.1016/j.eurpolymj.2017.10.021
Smith E, Abbott AP, Ryder KD (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Wang W, Sabo R, Mozuch M, Kersten P, Zhu J (2015) Jin Y (2015) Physical and mechanical properties of cellulose nanofibril films from bleached eucalyptus pulp by endoglucanase treatment and microfluidization. J Polym Environ 23:551–558. https://doi.org/10.1007/s10924-015-0726-7
Wang J, Gardner DJ, Stark NM, Bousfield DW, Tajvidi M, Cai Z (2018) Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustainable Chem Eng 6:49–70. https://doi.org/10.1021/acssuschemeng.7b03523
Yang X, Reid MS, Olsén P, Berglund LA (2020) Eco-friendly cellulose nanofibrils designed by nature: effects from preserving native state. ACS Nano 14:724–735. https://doi.org/10.1021/acsnano.9b07659
Yousefi H, Nishino T, Shakeri A, Faezipour M, Ebrahimi G, Kotera M (2013) Water-repellent all-cellulose nanocomposite using silane coupling treatment. J Adhes Sci Technol 27:1324–1334. https://doi.org/10.1080/01694243.2012.695954
Zhang Q, Vigier KDO, Royer V, Jérôme F (2012) Deep eutectic solvents: Syntheses, properties and applications. Chem Soc Rev 41:7108–7146
Zhang J, Luo N, Zhang X, Xu J, Yu J, He J, Zhang J (2016) All-cellulose nanocomposites reinforced with in situ retained cellulose nanocrystals during selective dissolution of cellulose in an ionic liquid. ACS Sustainable Chem Eng 4:4417–4423. https://doi.org/10.1021/acssuschemeng.6b01034
Zhu H, Fang Z, Preston C, Li Y, Hu L (2014) Transparent paper: Fabrications, properties, and device applications. Energy Environ Sci 7:269–287. https://doi.org/10.1039/c3ee43024c
Acknowledgments
The authors acknowledge the support from the Academy of Finland project “ACNF” (325276). FESEM imaging and XPS analysis were carried out with the support of the Centre for Material Analysis, University of Oulu, Finland. We gratefully thank the help of our laboratory staff.
Funding
Open access funding provided by University of Oulu including Oulu University Hospital. The research was supported by the Academy of Finland project “ACNF” (325276).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions from all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10570_2021_3863_MOESM1_ESM.docx
The FESEM images and XPS spectra of DES-modified films (CNF-DES and ACC-DES) and the corresponding XPS survey spectra are included in the supplementary material. This material is available online for authorized users.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lakovaara, M., Sirviö, J.A., Ismail, M.Y. et al. Hydrophobic modification of nanocellulose and all-cellulose composite films using deep eutectic solvent as a reaction medium. Cellulose 28, 5433–5447 (2021). https://doi.org/10.1007/s10570-021-03863-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03863-1