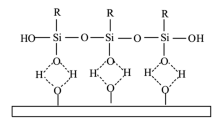
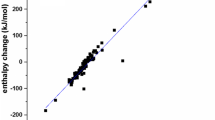
Abstract
The recent intensification of industrially produced cellulose nanocrystals (CNCs) and cellulose nanofibrils has positioned nanocelluloses as promising materials for many water-based products and applications. However, for nanocelluloses to move beyond solely an academic interest, a thorough understanding of their interaction with water-soluble polymers is needed. In this work, we address a conflicting trend in literature that suggests polyethylene glycol (PEG) adsorbs to CNC surfaces by comparing the adsorption behaviour of PEG with CNCs versus fumed silica. While PEG is known to have strong hydrogen bonding tendencies and holds water tightly, it is sometimes (we believe erroneously) presumed that PEG binds to cellulose through hydrogen bonding in aqueous media. To test this assumption, the adsorption of PEG to CNCs and fumed silica (both in the form of particle films and in aqueous dispersions) was examined using quartz crystal microbalance with dissipation, isothermal titration calorimetry, rheology and dynamic light scattering. For all PEG molecular weights (300–10,000 g/mol) and concentrations (100–10,000 ppm) tested, strong rapid adsorption was found with fumed silica, whereas no adsorption to CNCs was observed. We conclude that unlike silanols, the hydroxyl groups on the surface of CNCs do not readily hydrogen bond with the ether oxygen in the PEG backbone. As such, this work along with previous papermaking literature supports the opinion that PEG does not adsorb to cellulose surfaces.





Similar content being viewed by others
References
Aggebrandt LG, Samuelson O (1964) Penetration of water-soluble polymers into cellulose fibers. J Appl Polym Sci 8:2801–2812. doi:10.1002/app.1964.070080625
Araki J, Wada M, Kuga S (2001) Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 17:21–27. doi:10.1021/la001070m
Aulin C, Varga I, Claesson PM et al (2008) Buildup of polyelectrolyte multilayers of polyethyleneimine and microfibrillated cellulose studied by in situ dual-polarization interferometry and quartz crystal microbalance with dissipation. Langmuir 24:2509–2518. doi:10.1021/la7032884
Azizi Samir MAS, Alloin F, Sanchez JY, Dufresne A (2004) Cellulose nanocrystals reinforced poly(oxyethylene). Polymer (Guildf) 45:4149–4157. doi:10.1016/j.polymer.2004.03.094
Bardet R, Belgacem N, Bras J (2015) Flexibility and color monitoring of cellulose nanocrystal iridescent solid films using anionic or neutral polymers. ACS Appl Mater Interfaces 7:4010–4018. doi:10.1021/am506786t
Beck S, Bouchard J, Berry R (2012) Dispersibility in water of dried nanocrystalline cellulose. Biomacromol 13:1486–1494. doi:10.1021/bm300191k
Ben Azouz K, Ramires EC, Van den Fonteyne W et al (2012) Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macro Lett 1:236–240. doi:10.1021/mz2001737
Benselfelt T, Cranston ED, Ondaral S et al (2016) Adsorption of xyloglucan onto cellulose surfaces of different morphologies: an entropy-driven process. Biomacromol 17:2801–2811. doi:10.1021/acs.biomac.6b00561
Bhattacharyya L, Rohrer JS (eds) (2012) Applications of ion chromatography for pharmaceutical and biological products. Wiley, Hoboken
Biermann CJ (1996) Stock preparation and additives for papermaking. In: Handbook of pulping and papermaking, 2nd edn. Elsevier/Academic Press, London, pp 190–208. doi:10.1016/B978-012097362-0/50012-1
Boluk Y, Zhao L, Incani V (2012) Dispersions of nanocrystalline cellulose in aqueous polymer solutions: structure formation of colloidal rods. Langmuir 28:6114–6123. doi:10.1021/la2035449
Bouchard J, Méthot M, Fraschini C, Beck S (2016) Effect of oligosaccharide deposition on the surface of cellulose nanocrystals as a function of acid hydrolysis temperature. Cellulose 23:3555–3567. doi:10.1007/s10570-016-1036-5
Cabot Corporation (2016) Hydrophilic Fumed Silica. http://www.cabotcorp.com/solutions/products-plus/fumed-metal-oxides/hydrophilic. Accessed 11 Oct 2016
Cao Y, Zavaterri P, Youngblood J et al (2015) The influence of cellulose nanocrystal additions on the performance of cement paste. Cem Concr Compos 56:73–83. doi:10.1016/j.cemconcomp.2014.11.008
Changsarn S, Mendez JD, Shanmuganathan K et al (2011) Biologically inspired hierarchical design of nanocomposites based on poly(ethylene oxide) and cellulose nanofibers. Macromol Rapid Commun 32:1367–1372. doi:10.1002/marc.201100183
Cheng D, Wen Y, Wang L et al (2015) Adsorption of polyethylene glycol (PEG) onto cellulose nano-crystals to improve its dispersity. Carbohydr Polym 123:157–163. doi:10.1016/j.carbpol.2015.01.035
Chiad K, Stelzig SH, Gropeanu R et al (2009) Isothermal titration calorimetry: a powerful technique to quantify interactions in polymer hybrid systems. Macromolecules 42:7545–7552. doi:10.1021/ma9008912
de Cuadro P, Belt T, Kontturi KS et al (2015) Cross-linking of cellulose and poly(ethylene glycol) with citric acid. React Funct Polym 90:21–24. doi:10.1016/j.reactfunctpolym.2015.03.007
De France KJ, Chan KJW, Cranston ED, Hoare T (2016) Enhanced mechanical properties in cellulose nanocrystal–poly(oligoethylene glycol methacrylate) injectable nanocomposite hydrogels through control of physical and chemical cross-linking. Biomacromol 17:649–660. doi:10.1021/acs.biomac.5b01598
Devanand K, Selser JC (1991) Asymptotic behavior and long-range interactions in aqueous solutions of poly (ethylene oxide). Macromolecules 24:5943–5947. doi:10.1021/ma00022a008
Dong XM, Kimura T, Revol J-F, Gray DG (1996) Effects of ionic strength on the isotropic–chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 12:2076–2082. doi:10.1021/la950133b
Edgar CD, Gray DG (2002) Influence of dextran on the phase behavior of suspensions of cellulose nanocrystals. Macromolecules 35:7400–7406. doi:10.1021/ma0204195
Eisenlauer J, Killmann E, Korn M (1980) Stability of colloidal silica (aerosil) hydrosols. II. Influence of the pH value and the adsorption of polyethylene glycols. J Colloid Interface Sci 74:120–135. doi:10.1016/0021-9797(80)90176-9
Eronen P, Junka K, Laine J, Österberg M (2011) Interaction between water-soluble polysaccharides and native nanofibrillar cellulose thin films. BioResources 6:4200–4217
Esumi K, Iitaka M, Koide Y (1998) Simultaneous adsorption of poly(ethylene oxide) and cationic surfactant at the silica/water interface. J Colloid Interface Sci 208:178–182. doi:10.1006/jcis.1998.5819
Esumi K, Nakaie Y, Sakai K, Torigoe K (2001) Adsorption of poly(ethyleneglycol) and poly(amidoamine)dendrimer from their mixtures on alumina/water and silica/water interfaces. Colloids Surfaces A Physicochem Eng Asp 194:7–12. doi:10.1016/S0927-7757(00)00788-3
Fleer GJ, Cohen Stuart MA, Scheutjens JMHM et al (1998) Polymers at interfaces. Chapman & Hall, Illistrate
Gårdebjer S, Andersson M, Engström J et al (2016) Using ansen solubility parameters to predict the dispersion of nano-particles in polymeric films. Polym Chem 7:1756–1764. doi:10.1039/C5PY01935D
Gray DG, Mu X (2015) Chiral nematic structure of cellulose nanocrystal suspensions and films; polarized light and atomic force microscopy. Materials (Basel) 8:7873–7888. doi:10.3390/ma8115427
Grishkewich N, Mohammed N, Tang J, Tam KC (2017) Recent advances in the application of cellulose nanocrystals. Curr Opin Colloid Interface Sci 29:32–45. doi:10.1016/j.cocis.2017.01.005
Gustafsson E, Pelton R, Wågberg L (2016) Rapid development of wet adhesion between carboxymethylcellulose modified cellulose surfaces laminated with polyvinylamine adhesive. ACS Appl Mater Interfaces 8:24161–24167. doi:10.1021/acsami.6b05673
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500. doi:10.1021/cr900339w
Hasanzadeh M, Mottaghitalab V, Rezaei M (2015) Rheological and viscoelastic behavior of concentrated colloidal suspensions of silica nanoparticles: a response surface methodology approach. Adv Powder Technol 26:1570–1577. doi:10.1016/j.apt.2015.08.011
Hatton FL, Malmström E, Carlmark A (2015) Tailor-made copolymers for the adsorption to cellulosic surfaces. Eur Polym J 65:325–339. doi:10.1016/j.eurpolymj.2015.01.026
He X, Male KB, Nesterenko PN et al (2013) Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl Mater Interfaces 5:8796–8804. doi:10.1021/am403222u
Hoeger I, Rojas OJ, Efimenko K et al (2011) Ultrathin film coatings of aligned cellulose nanocrystals from a convective-shear assembly system and their surface mechanical properties. Soft Matter 7:1957. doi:10.1039/c0sm01113d
Holappa S, Kontturi KS, Salminen A et al (2013) Adsorption of hydrophobically end-capped poly(ethylene glycol) on cellulose. Langmuir 29:13750–13759. doi:10.1021/la402494m
Honorato-Rios C, Kuhnhold A, Bruckner J et al (2016) Equilibrium liquid crystal phase diagrams and detection of kinetic arrest in cellulose nanocrystal suspensions. Front Mater 3:1–13. doi:10.3389/fmats.2016.00021
Hu Z, Cranston ED, Ng R, Pelton R (2014) Tuning cellulose nanocrystal gelation with polysaccharides and surfactants. Langmuir 30:2684–2692. doi:10.1021/la404977t
Hu Z, Patten T, Pelton R, Cranston ED (2015) Synergistic stabilization of emulsions and emulsion gels with water-soluble polymers and cellulose nanocrystals. ACS Sustain Chem Eng 3:1023–1031. doi:10.1021/acssuschemeng.5b00194
Hu Z, Xu R, Cranston ED, Pelton RH (2016) Stable aqueous foams from cellulose nanocrystals and methyl cellulose. Biomacromol 17:4095–4099. doi:10.1021/acs.biomac.6b01641
Huang L, Nishinari K (2001) Interaction between poly(ethylene glycol) and water as studied by differential scanning calorimetry. J Polym Sci Part B: Polym Phys 39:496–506. doi:10.1002/1099-0488(20010301)39:5<496:AID-POLB1023>3.0.CO;2-H
Hubbe MA (2007) Flocculation and redispersion of cellulosic fiber suspensions: a review of effects of hydrodynamic shear and polyelectrolytes. BioResources 2:296–331
Hubbe MA, Nanko H, McNeal MR (2009) Retention aid polymer interactions with cellulosic surfaces and suspensions: a review. BioResources 4:850–906
Hyde EDER, Seyfaee A, Neville F, Moreno-Atanasio R (2016) Colloidal silica particle synthesis and future industrial manufacturing pathways: a review. Ind Eng Chem Res 55:8891–8913. doi:10.1021/acs.iecr.6b01839
Ishimaru Y, Lindström T (1984) Adsorption of water-soluble, nonionic polymers onto cellulosic fibers. J Appl Polym Sci 29:1675–1691. doi:10.1002/app.1984.070290521
Kalashnikova I, Bizot H, Cathala B, Capron I (2012) Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromol 13:267–275. doi:10.1021/bm201599j
Kalashnikova I, Bizot H, Bertoncini P et al (2013) Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 9:952. doi:10.1039/c2sm26472b
Kalasin S, Santore MM (2016) Near-surface motion and dynamic adhesion during silica microparticle capture on a polymer (solvated PEG) brush via hydrogen bonding. Macromolecules 49:334–343. doi:10.1021/acs.macromol.5b01977
Kargl R, Mohan T, Bračič M et al (2012) Adsorption of carboxymethyl cellulose on polymer surfaces: evidence of a specific interaction with cellulose. Langmuir 28:11440–11447. doi:10.1021/la302110a
Karimi K, Taherzadeh MJ (2016) A critical review on analysis in pretreatment of lignocelluloses: degree of polymerization, adsorption/desorption, and accessibility. Bioresour Technol 203:348–356. doi:10.1016/j.biortech.2015.12.035
Khandavalli S, Rothstein JP (2014) Extensional rheology of shear-thickening fumed silica nanoparticles dispersed in an aqueous polyethylene oxide solution. J Rheol (NY) 58:411–431. doi:10.1122/1.4864620
Kim SY, Meyer HW, Saalwächter K, Zukoski CF (2012) Polymer dynamics in PEG-silica nanocomposites: effects of polymer molecular weight, temperature and solvent dilution. Macromolecules 45:4225–4237. doi:10.1021/ma300439k
Klemm D, Kramer F, Moritz S et al (2011) Nanocelluloses: a new family of nature-based materials. Angew Chemie Int Ed 50:5438–5466. doi:10.1002/anie.201001273
Kloser E, Gray DG (2010) Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 26:13450–13456. doi:10.1021/la101795s
Knop K, Hoogenboom R, Fischer D, Schubert US (2010) Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chemie Int Ed 49:6288–6308. doi:10.1002/anie.200902672
Kondo T, Sawatari C (1994) Intermolecular hydrogen bonding in cellulose/poly(ethylene oxide) blends: thermodynamic examination using 2,3-di-O- and 6-O-methylcelluloses as cellulose model compounds. Polymer (Guildf) 35:4423–4428. doi:10.1016/0032-3861(94)90102-3
Kontturi E, Johansson L, Kontturi K (2007) Cellulose nanocrystal submonolayers by spin coating. Langmuir 23:9674–9680
Kontturi KS, Biegaj K, Mautner A et al (2017) Noncovalent surface modification of cellulose nanopapers by adsorption of polymers from aprotic solvents. Langmuir 33:5707–5712. doi:10.1021/acs.langmuir.7b01236
Laine J, Lindström T, Glad-Nordmark G, Risinger G (2000) Studies on topochemical modification of cellulosic fibres. Part 1. Chemical conditions for the attachment of carboxymethyl cellulose onto fibres. Nord Pulp Pap Res J 15:520–526. doi:10.3183/NPPRJ-2000-15-05-p520-526
Leung K, Nielsen I, Criscenti L (2009) Elucidating the bimodal acid–base behavior of the water–silica interface from first principles. J Am Chem Soc 131:18358–18365. doi:10.1021/ja906190t
Li Y, Liu H, Song J et al (2011) Adsorption and association of a symmetric PEO–PPO–PEO triblock copolymer on polypropylene, polyethylene, and cellulose surfaces. ACS Appl Mater Interfaces 3:2349–2357. doi:10.1021/am200264r
Lindström T, Glad-Nordmark G (1983) Selective adsorption, flocculation, and fractionation of wood pulps with polyethyleneoxide. J Colloid Interface Sci 94:404–411. doi:10.1016/0021-9797(83)90280-1
Lindström T, Glad-Nordmark G (1984) Flocculation of latex and cellulose dispersions by means of transient polymer networks. Colloids Surf 8:337–351. doi:10.1016/0166-6622(84)80128-6
Liu H, Xiao H (2008) Adsorption of poly(ethylene oxide) with different molecular weights on the surface of silica nanoparticles and the suspension stability. Mater Lett 62:870–873. doi:10.1016/j.matlet.2007.06.079
Lopez M, Bizot H, Chambat G et al (2010) Enthalpic studies of xyloglucan–cellulose interactions. Biomacromol 11:1417–1428. doi:10.1021/bm1002762
Lu A, Hemraz U, Khalili Z, Boluk Y (2014) Unique viscoelastic behaviors of colloidal nanocrystalline cellulose aqueous suspensions. Cellulose 21:1239–1250. doi:10.1007/s10570-014-0173-y
Madathingal RR, Wunder SL (2011) Confinement effects of silica nanoparticles with radii smaller and larger than Rg of adsorbed poly(ethylene oxide). Macromolecules 44:2873–2882. doi:10.1021/ma1021693
Malmsten M, Linse P, Cosgrove T (1992) Adsorption of PEO–PPO–PEO block copolymers at silica. Macromolecules 25:2474–2481. doi:10.1021/ma00035a028
Mariano M, El Kissi N, Dufresne A (2014) Cellulose nanocrystals and related nanocomposites: review of some properties and challenges. J Polym Sci Part B: Polym Phys 52:791–806. doi:10.1002/polb.23490
Martin C, Jean B (2014) Nanocellulose/polymer multilayered thin films: tunable architectures towards tailored physical properties. Nord Pulp Pap Res J 29:19–30
Mathur S, Moudgil BM (1997) Adsorption mechanism(s) of poly(ethylene oxide) on oxide surfaces. J Colloid Interface Sci 196:92–98. doi:10.1006/jcis.1997.5192
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994. doi:10.1039/c0cs00108b
Niinivaara E, Faustini M, Tammelin T, Kontturi E (2015) Water vapor uptake of ultrathin films of biologically derived nanocrystals: quantitative assessment with quartz crystal microbalance and spectroscopic ellipsometry. Langmuir 31:12170–12176. doi:10.1021/acs.langmuir.5b01763
Noroozi N, Grecov D, Shafiei-Sabet S (2013) Estimation of viscosity coefficients and rheological functions of nanocrystalline cellulose aqueous suspensions. Liq Cryst 41:56–66. doi:10.1080/02678292.2013.834081
Oguzlu H, Boluk Y (2017) Interactions between cellulose nanocrystals and anionic and neutral polymers in aqueous solutions. Cellulose 24:131–146. doi:10.1007/s10570-016-1096-6
Oguzlu H, Danumah C, Boluk Y (2016) The role of dilute and semi-dilute cellulose nanocrystal (CNC) suspensions on the rheology of carboxymethyl cellulose (CMC) solutions. Can J Chem Eng 94:1841–1847. doi:10.1002/cjce.22597
Oksman K, Aitomäki Y, Mathew AP et al (2016) Review of the recent developments in cellulose nanocomposite processing. Compos Part A Appl Sci Manuf 83:2–18. doi:10.1016/j.compositesa.2015.10.041
Pelton RH, Allen LH, Nugent HM (1980) Survey of potential retention aids for newsprint manufacture. Pulp Pap Can 81(54–56):58
Pelton RH, Allen LH, Nugent HM (1981) Novel dual-polymer retention aids for newsprint and groundwood specialties. Tappi 64:89–92
Raghavan SR, Walls HJ, Khan SA (2000) Rheology of silica dispersions in organic liquids: new evidence for solvation forces dictated by hydrogen bonding. Langmuir 16:7920–7930. doi:10.1021/la991548q
Reid MS, Villalobos M, Cranston ED (2016) Cellulose nanocrystal interactions probed by thin film swelling to predict dispersibility. Nanoscale 8:12247–12257. doi:10.1039/C6NR01737A
Reid MS, Kedzior SA, Villalobos M, Cranston ED (2017a) Effect of ionic strength and surface charge density on the kinetics of cellulose nanocrystal thin film swelling. Langmuir. doi:10.1021/acs.langmuir.7b01740
Reid MS, Villalobos M, Cranston ED (2017b) Benchmarking cellulose nanocrystals: from the laboratory to industrial production. Langmuir 33:1583–1598. doi:10.1021/acs.langmuir.6b03765
Reid MS, Villalobos M, Cranston ED (2017c) The role of hydrogen bonding in non-ionic polymer adsorption to cellulose nanocrystals and silica colloids. Curr Opin Colloid Interface Sci 29:76–82. doi:10.1016/j.cocis.2017.03.005
Revol JF, Bradford H, Giasson J et al (1992) Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int J Biol Macromol 14:170–172
Rubio J, Kitchener J (1976) The mechanism of adsorption of poly(ethylene oxide) flocculant on silica. J Colloid Interface Sci 57:132–142. doi:10.1016/0021-9797(76)90182-X
Saigal T, Riley JK, Golas PL et al (2013) Poly(ethylene oxide) star polymer adsorption at the silica/aqueous interface and displacement by linear poly(ethylene oxide). Langmuir 29:3999–4007. doi:10.1021/la305085a
Shafiei-Sabet S, Hamad WY, Hatzikiriakos SG (2012) Rheology of nanocrystalline cellulose aqueous suspensions. Langmuir 28:17124–17133. doi:10.1021/la303380v
Shafiei-Sabet S, Hamad WY, Hatzikiriakos SG (2014) Ionic strength effects on the microstructure and shear rheology of cellulose nanocrystal suspensions. Cellulose 21:3347–3359. doi:10.1007/s10570-014-0407-z
Stone JE, Scallan AM (1967) The effect of component removal upon the porous structure of the cell wall of wood. II. Swelling in water and the fiber saturation point. Tappi 50:496–501. doi:10.1002/polc.5070110104
Sundman O (2014) Adsorption of four non-ionic cellulose derivatives on cellulose model surfaces. Cellulose 21:115–124. doi:10.1007/s10570-013-0105-2
Tarkow H, Feist WC, Southerland CF (1966) Interaction of wood with polymeric materials size. For Prod J 16:61–65
Trey SM, Netrval J, Berglund L, Johansson M (2010) Electron-beam-initiated polymerization of poly(ethylene glycol)-based wood impregnants. ACS Appl Mater Interfaces 2:3352–3362. doi:10.1021/am100778q
Ureña-Benavides EE, Ao G, Davis VA, Kitchens CL (2011) Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 44:8990–8998. doi:10.1021/ma201649f
Utsuno K, Uludaǧ H (2010) Thermodynamics of polyethylenimine-DNA binding and DNA condensation. Biophys J 99:201–207. doi:10.1016/j.bpj.2010.04.016
Viet D, Beck-Candanedo S, Gray DG (2006) Dispersion of cellulose nanocrystals in polar organic solvents. Cellulose 14:109–113. doi:10.1007/s10570-006-9093-9
Wågberg L (2000) Polyelectrolyte adsorption onto cellulose fibres—a review. Nord Pulp Pap Res J 15:586–597. doi:10.3183/NPPRJ-2000-15-05-p586-597
Xiao H, Pelton RH, Archie H (1996) Novel rention aids for mechanical pulps. Tappi J 79:129–135
Xu X, Liu F, Jiang L et al (2013) Cellulose nanocrystals vs. Cellulose nanofibrils: a comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl Mater Interfaces 5:2999–3009. doi:10.1021/am302624t
Yu HY, Zhang DZ, Lu FF, Yao J (2016) New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain Chem Eng 4:2632–2643. doi:10.1021/acssuschemeng.6b00126
Zaman AA (2000) Effect of polyethylene oxide on the viscosity of dispersions of charged silica particles: interplay between rheology, adsorption, and surface charge. Colloid Polym Sci 278:1187–1197. doi:10.1007/s003960000385
Zhang Q, Archer LA (2002) Poly(ethylene oxide)/silica nanocomposites: structure and rheology. Langmuir 18:10435–10442. doi:10.1021/la026338j
Acknowledgments
Funding from the Natural Sciences and Engineering Research Council of Canada, Industrial Postgraduate Scholarship program sponsored by Cabot Corporation is gratefully acknowledged. We additionally acknowledge Cabot Corporation for their donation of CAB-O-SIL® M-5 fumed silica. Professors R. Pelton, A. Guarne, R. Epand and J. Moran-Mirabal are thanked for sharing equipment to characterize CNCs. Additionally, the Biointerfaces Institute and the Brockhouse Institute for Materials Research at McMaster University are acknowledged for support and equipment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reid, M.S., Marway, H.S., Moran-Hidalgo, C. et al. Comparison of polyethylene glycol adsorption to nanocellulose versus fumed silica in water. Cellulose 24, 4743–4757 (2017). https://doi.org/10.1007/s10570-017-1482-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1482-8