Abstract
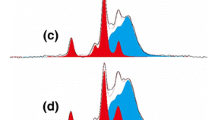
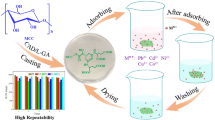
Cellulose/chitosan composites were successfully prepared in a new and basic-based solvent system, ethylene diamine/potassium thiocyanate (EDA/KSCN), by dissolving cellulose and chitosan in 70/30 (w/w) EDA/KSCN at −19 °C, and then coagulating in methanol. Wide angle X-ray diffraction studies revealed that the EDA/KSCN solvent system is capable of disrupting the hydrogen bonds in both cellulose and chitosan and increase the amorphous regions. Stability tests proved that the composites are stable in acidic aqueous solution due to the hydrogen bonds formed between cellulose and chitosan. This is the first time to dissolve chitosan in a basic-based solvent system and prepare cellulose/chitosan composites in a straightforward way. The adsorption of heavy metal ions (Cu2+, Cd2+, and Pb2+) onto the cellulose/chitosan composites was investigated. The adsorption capacity is highly dependent on pH and the maximum metal uptake was obtained at pH 5.0. Increasing initial metal concentration enhanced the diffusion of metal ions to the composite surface and therefore the metal removal efficiency. Higher percentage of chitosan in the composites also led to higher metal adsorption. The results indicated that the prepared cellulose/chitosan (1:1) composite can adsorb 0.53 mmol/g Cu2+, 0.28 mmol/g Cd2+ and 0.16 mmol/g Pb2+ ions at pH 5.0. The Freundlich model and the pseudo-second-order model were in good agreement with the adsorption isotherms and kinetics, respectively. X-ray photoelectron spectroscopy studies indicated that the binding of heavy metal ions is attributed to the nitrogen atoms of amino groups in chitosan. The composites can be reused for metal removal.









Similar content being viewed by others
References
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167(1):482–488
Bassi R, Prasher SO, Simpson B (2000) Removal of selected metal ions from aqueous solutions using chitosan flakes. Sep Sci Technol 35(4):547–560
Bessbousse H, Rhlalou T, Verchère J-F, Lebrun L (2008) Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly (ethyleneimine) in a poly (vinyl alcohol) matrix. J Membr Sci 307(2):249–259
Evans JR, Davids WG, MacRae JD, Amirbahman A (2002) Kinetics of cadmium uptake by chitosan-based crab shells. Water Res 36(13):3219–3226
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38(1):43–74
Guibal E, Cambe S, Bayle S, Taulemesse JM, Vincent T (2013) Silver/chitosan/cellulose fibers foam composites: from synthesis to antibacterial properties. J Colloid Interface Sci 393:411–420
Ho Y, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76(4):332–340
Jin L, Bai R (2002) Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 18(25):9765–9770
Kannamba B, Reddy KL, AppaRao B (2010) Removal of Cu (II) from aqueous solutions using chemically modified chitosan. J Hazard Mater 175(1):939–948
Ku Y, Jung I-L (2001) Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res 35(1):135–142
Lee ST, Mi FL, Shen YJ, Shyu SS (2001) Equilibrium and kinetic studies of copper(II) ion uptake by chitosan-tripolyphosphate chelating resin. Polymer 42(5):1879–1892
Li N, Bai R (2005) Copper adsorption on chitosan–cellulose hydrogel beads: behaviors and mechanisms. Sep Purif Technol 42(3):237–247
Liu Z, Wang H, Liu C, Jiang Y, Yu G, Mu X, Wang X (2012) Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem Commun 48(59):7350–7352
Liu Z, Sun X, Hao M, Huang C, Xue Z, Mu T (2015) Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr Polym 117:99–105
Miya M, Iwamoto R, Mima S (1984) FT-IR study of intermolecular interactions in polymer blends. J Polym Sci Polym Phys Edit 22(6):1149–1151
Mudhoo A, Garg VK, Wang S (2012) Removal of heavy metals by biosorption. Environ Chem Lett 10(2):109–117
Nadeem M, Mahmood A, Shahid S, Shah S, Khalid A, McKay G (2006) Sorption of lead from aqueous solution by chemically modified carbon adsorbents. J Hazard Mater 138(3):604–613
Ngah W, Fatinathan S (2008) Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem Eng J 143(1):62–72
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol 99(15):6709–6724
Onsosyen E, Skaugrud O (1990) Metal recovery using chitosan. J Chem Technol Biotechnol 49(4):395–404
Osifo PO, Webster A, van der Merwe H, Neomagus HW, van der Gun MA, Grant DM (2008) The influence of the degree of cross-linking on the adsorption properties of chitosan beads. Bioresour Technol 99(15):7377–7382
Ozaki H, Sharma K, Saktaywin W (2002) Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: effects of interference parameters. Desalination 144(1):287–294
Park GI, Park HS, Woo SI (1999) Influence of pH on the adsorption of uranium ions by oxidized activated carbon and chitosan. Sep Sci Technol 34(5):833–854
Ravi Kumar MN (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Reddad Z, Gérente C, Andrès Y, Ralet M-C, Thibault J-F, Cloirec PL (2002a) Ni (II) and Cu (II) binding properties of native and modified sugar beet pulp. Carbohydr Polym 49(1):23–31
Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002b) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36(9):2067–2073
Samuels RJ (1981) Solid state characterization of the structure of chitosan films. J Polym Sci Polym Phys Edit 19(7):1081–1105
Striegel AM (2003) Advances in the understanding of the dissolution mechanism of cellulose in DMAc/LiCl. J Chil Chem Soc 48(1):73–77
Sun X, Peng B, Ji Y, Chen J, Li D (2009) Chitosan (chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J 55(8):2062–2069
Ünlü N, Ersoz M (2006) Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater 136(2):272–280
Vieira RS, Oliveira MLM, Guibal E, Rodríguez-Castellón E, Beppu MM (2011) Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: an XPS investigation of mechanism. Colloids Surf A 374(1):108–114
Wan Ngah W, Hanafiah M (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99(10):3935–3948
Wan Ngah W, Endud C, Mayanar R (2002) Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym 50(2):181–190
Wang J, Wei L, Ma Y, Li K, Li M, Yu Y, Wang L, Qiu H (2013) Collagen/cellulose hydrogel beads reconstituted from ionic liquid solution for Cu (II) adsorption. Carbohydr Polym 98(1):736–743
Xiao M, Frey MW (2007) The role of salt on cellulose dissolution in ethylene diamine/salt solvent systems. Cellulose 14(3):225–234
Xiao M, Frey MW (2008) Rheological studies of the interactions in cellulose/ethylene diamine/salt systems. J Polym Sci Part B Polym Phys 46(21):2326–2334
Xiao M, Frey MW (2009) Study of cellulose/ethylene diamine/salt systems. Cellulose 16(3):381–391
Zhou D, Zhang L, Zhou J, Guo S (2004) Cellulose/chitin beads for adsorption of heavy metals in aqueous solution. Water Res 38(11):2643–2650
Zhou D, Zhang L, Guo S (2005) Mechanisms of lead biosorption on cellulose/chitin beads. Water Res 39(16):3755–3762
Acknowledgments
This work was supported by the Administration of Quality and Technology Supervision of Guangzhou Municipality, Guangzhou, China [Grant Number 2014ZZ09].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, M., Hu, J. Cellulose/chitosan composites prepared in ethylene diamine/potassium thiocyanate for adsorption of heavy metal ions. Cellulose 24, 2545–2557 (2017). https://doi.org/10.1007/s10570-017-1287-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1287-9