Abstract
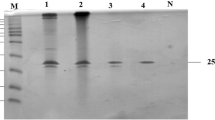
Thermostable xylanase isoforms T70 and T90 were purified and characterized from the xerophytic Opuntia vulgaris plant species. The enzyme was purified to homogeneity employing three consecutive steps. The purified T70 and T90 isoforms yielded a final specific activity 134.0 and 150.8 U mg−1 protein, respectively. The molecular mass of these isoforms was determined to be 27 kDa. The optimum pH for the T70 and T90 xylanase isoforms was 5.0 and the temperature for optimal activity was 70 and 90 °C, respectively. The Km value of T70 and T90 enzyme isoforms was 3.49, 2.1 mg ml−1, respectively when oat spelt xylan was used as a substrate. The T70 had a Vmax of 10.4 μmol min−1 mg−1, and T90 had a Vmax of 8.9 μmol min−1 mg−1, respectively. In the presence of 10 mM Co2+, and Mn2+ the activity of T70 and T90 isoforms increased, where as 90 % inhibition was noted with of the use 10 mM Hg2+, Cd2+, Cu2+, Zn2+ while partial inhibition was observed in the presence of Fe3+, Ni2+, Ca2+and Mg2+. The T70 and T90 isoforms retained nearly 50 % activity in the presence of 2.0 M urea, while use of 40 mM SDS lowered the activity nearly 38–41 %. The substrate specificity of both T70 and T90 isoforms showed maximum activity for oat spelt xylan. Western blot, immunodiffusion, and in vitro inhibition assays confirmed reactivity of the T90 isoform with polyclonal anti-T90 antibody raised in rabbit, as well as cross-reactivity of the antibody with the T70 xylanase isoform.




Similar content being viewed by others
References
Antranikian G (1997) Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol 17:39–67
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Biely P, Markovic O, Mislovic D (1985) Sensitive detection of endo-1,4-β-glucanases and endo-1,4-β-xylanases in gels. Anal Biochem 144:147–151
Blanco A, Pastor FIJ (1993) Characterization of cellulase-free xylanases from the newly isolated Bacillus sp. strain BP-23. Can J Microbiol 39:1162–1166
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Cannio R, Di Prizito N, Rossi Alessandra Morana M (2004) A xylan-degrading strain of Sulfolobus solfataricus: isolation and characterization of the xylanase activity. Extremophiles 8:117–124
Dekker RFH, Richards GN (1975) Purification, properties, and mode of action of hemicellulase I produced by Ceratocystis paradoxa. Carbohydr Res 39:97–114
Fushinobu S, Ito K, Konno M, Wakagi T, Matsuzawa H (1998) Crystallographic and mutational analyses of an extremely acidophilic and acid-stable xylanase: biased distribution of acidic residues and importance of Asp37 for catalysis at low pH. Protein Eng 11:1121–1128
Iefuji H, Chino M, Kato M, Iimura Y (1996) Acid xylanase from yeast Cryptococcus sp. S-2: purification, characterization, cloning, and sequencing. Biosci Biotechnol Biochem 60:1331–1338
Jänis J, Turunen O, Leisola M, Derrick PJ, Rouvinen J, Vainiotalo P (2004) Characterization of mutant xylanases using fourier transform ion cyclotron resonance mass spectrometry: stabilizing contributions of disulfide bridges and N-terminal extensions. Biochemistry (Moscow) 43:9556–9566
Joseleau JP, Comtat J, Ruel K (1992) Chemical structure of xylans and their interactions in the plant cell walls. In: Visser J, Beldman G, van Someren MAK, Voragen AGJ (eds) Xylans and xylanases, Elsevier, Amsterdam, pp 1–15
Khandeparkar R, Bhosle NB (2006) Purification and characterization of thermoalkalophilic xylanase isolated from the Enterobacter sp. MTCC 5112. Res Microbiol 157:315–325
Krisana A, Rutchadaporn S, Jarupan G, Lily E, Sutipa T, Kanyawim K (2005) Endo-1,4- β-xylanase B from Aspergillus cf. niger BCC14405 isolated in Thailand: purification, characterization and gene isolation. J Biochem Mol Biol 38:17–23
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee CC, Smith M, Kibblewhite-Accinelli RE, Williams TG, Wagschal K, Robertson GH, Wong DWS (2006) Isolation and characterization of a cold-active xylanase enzyme from Flavobacterium sp. Curr Microbiol 52:112–116
Leskinen S, Mäntylä A, Fagerström R, Vehmaanperä J, Lantto R, Paloheimo M, Suominen P (2005) Thermostable xylanases, Xyn10A and Xyn11A, from the actinomycete Nonomuraea flexuosa: isolation of the genes and characterization of recombinant Xyn11A polypeptides produced in Trichoderma reesei. Appl Microbiol Biotechnol 67:495–505
Mata G, Savoie JM (1998) Extracellular enzyme activities in six Lentinula edodes strains during cultivation in wheat straw. World J Microbiol Biotechnol 14:513–519
Mendicuti Castro LP, Trejo-Aguilar BA, Osorio GA (1997) Thermostable xylanases produced at 37 °C and 45 °C by a thermotolerant Aspergillus strain. FEMS Microbiol Lett 146:97–102
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nakamura S, Wakabayashi K, Nakai R, Aono R, Horikoshi K (1993) Purification and some properties of an alkaline xylanase from alkaliphilic Bacillus sp. strain 41 M–1. Appl Environ Microbiol 59:2311–2316
Ravikumar S, Vikramathithan J, Srikumar K (2011) Purification and characterization of a novel thermostable xylose isomerase from Opuntia vulgaris mill. Appl Biochem Biotechnol 164:593–603
Royer JC, Nakas JP (1991) Purification and characterization of two xylanases from Trichoderma longibrachiatum. Eur J Biochem 202:521–529
Ruiz-Arribas A, Santamaria RI, Zhadan GG, Villar E, Shnyrov VL (1994) Differential scanning calorimetric study of the thermal stability of xylanase from Streptomyces halstedii JM8. Biochemistry 33:13787–13791
Shyamala S, Ravikumar S, Vikramathithan J, Srikumar K (2011) Isolation, purification, and characterization of two thermostable endo-1,4-β-D-glucanase forms from Opuntia vulgaris. Appl Microbiol Biotechnol 165:1597–1610
Subramaniyan S, Prema P (2000) Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol Lett 183:1–7
Tachaapaikoon C, Kyu KL, Ratanakhanokchai K (2006) Purification of xylanase from alkaliphilic Bacillus sp. K-8 by using corn husk column. Process Biochem 41:2441–2445
Timell TE (1967) Recent progress in the chemistry of wood hemicelluloses. Wood Sci Technol 1:45–70
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and application. Proc Natl Acad Sci USA 76:4350
Viikari L, Kantelinen A, Sundquist J, Linko M (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev 13:335–350
Winterhalter C, Liebl W (1995) Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol 61:1810–1815
Wong KK, Tan LU, Saddler JN (1988) Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Zverlov V, Piotukh K, Dakhova O, Velikodvorskaya G, Borriss R (1996) The multidomain xylanase A of the hyperthermophilic bacterium Thermotoga neapolitana is extremely thermoresistant. Appl Microbiol Biotechnol 45:245–247
Acknowledgments
The authors gratefully acknowledge the University Grants Commission, India; Department of Science & Technology (DST) and the DST-Fund for Improvement of Science & Technology Infrastructure in Universities and Higher Educational Institutions (FIST) program, New Delhi, India for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vikramathithan, J., Ravikumar, S., Muthuraman, P. et al. Purification and biochemical characterization of two major thermophilic xylanase isoforms (T70 and T90) from xerophytic Opuntia vulgaris plant spp.. Cellulose 19, 1373–1383 (2012). https://doi.org/10.1007/s10570-012-9690-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9690-8