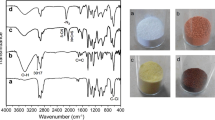
Abstract
The ionic liquid 1-N-butyl-3-methylimidazolium chloride ([C4mim]+Cl−) was investigated as reaction media for the homogeneous acylation of cellulose with 2-furoyl chloride in the presence of pyridine. The preparation of cellulose furoate depending on the reaction conditions, the cellulose type and the pyridine content was studied. Cellulose furoates with a degree of substitution in the range from 0.46 to 3.0 were accessible, i.e., under mild conditions, with a low excess of reagent and in a short reaction time. The products were characterized by elemental analysis, perpropionylation, 1H- and 13C NMR spectroscopy and FTIR spectroscopy.



Similar content being viewed by others
Abbreviations
- AGU:
-
Anhydroglucose unit
- CDI:
-
N,N′-carbonyldiimidazole
- [C4mim]+Cl− :
-
1-N-Butyl-3-methylimidazolium chloride
- DMSO:
-
Dimethyl sulfoxide
- DP:
-
Degree of polymerization
- DS:
-
Degree of substitution
- FC:
-
2-Furoyl chloride
- IL:
-
Ionic liquid
References
Ass BA, Frollini E, Heinze T (2004) Studies on the homogeneous acetylation of cellulose in the novel solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate. Macromol Biosci 4:1008–1013
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301–306
Chatterjee PK, Stanonis DJ (1966) Furoylation of cellulose. J Polym Sci 4:434–437
Cadotte JE, Rozelle LT, Petersen RJ et al (1970) Water transport across ultrathin membranes of mixed cellulose ester and ether derivatives. Appl Polym Symp 13:73–83
Ciacco GT, Liebert T, Frollini E et al (2003) Application of the solvent dimethyl sulfoxide/tetrabutyl ammonium fluoride trihydrate as reaction medium for the homogeneous acylation of Sisal cellulose. Cellulose 10:125–132
Dawsey TR, McCormick CL (1990) The lithium chloride/dimethylacetamide solvent for cellulose: a literature review. J Macromol Sci – Rev Macromol Chem Phys C30:405–440
Heinze T (2004) Chemical functionalization of cellulose. In: Dumitriu S, Dekker M (eds), Polysaccharide: structural diversity and functional versatility, 2nd edn, New York
Heinze T, Liebert T (2001) Unconventional methods in cellulose functionalization. Prog Polym Sci 26:1689–1762
Heinze T, Dicke R, Koschella A et al (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium for cellulose functionalization. Macromol Biosci 5:520–525
Hon DN-S, Yan H (2001a) Cellulose furoate. I. Synthesis in homogeneous and heterogeneous systems. J Appl Polym Sci 81:2649–2655
Hon DN-S, Yan H (2001b) Cellulose furoate. II. Characterization. J Appl Polym Sci 82:243–252
Hon DN-S, Yan H (2001c) Cellulose furoate. III. Properties and applications. J Appl Polym Sci 82:243–257
Hussain M, Liebert T, Heinze T (2004) Acylation of cellulose with N,N`-carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol Rapid Commun 25:916–920
Köhler S, Heinze T (2007) New solvents for cellulose: dimethyl sulfoxide/ammonium fluorides. Macromol Biosci 7:307–314
Liebert T, Heinze T (2005) Tailored cellulose esters: synthesis and structure determination. Biomacromolecules 6:333–340
Samaranayake G, Glasser W (1993) Cellulose derivatives with low DS. I: a novel acylation system. Carbohydr Polym 22:1–7
Schlufter K, Schmauder H-P, Dorn S et al (2006) Efficient homogeneous chemical modification of bacterial cellulose in the ionic liquid 1-N-butyl-3-methylimidazolium chloride. Macromol Rapid Commun 27:1670–1676
Singh S, Hinojosa O, Arthur JC (1970) ESR study of intramolecular energy transfer in the radiolysis of cellulose furoates. J Appl Polym Sci 14:1591–1596
Spear SK, Holbrey JD, Rogers R (2004) Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules 5:1379–1384
Swatloski RP, Spear SK, Holbrey JD et al (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Wu J, Zhang J, Zhang H et al (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266–268
Acknowledgements
The financial support of the ‘‘Forschungsvereinigung Werkstoffe aus Nachwachsenden Rohstoffen e.V. Rudolstadt’’ is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Thomas Heinze is the member of the European Polysaccharide Network of Excellence (EPNOE), www.epnoe.eu
Rights and permissions
About this article
Cite this article
Köhler, S., Heinze, T. Efficient synthesis of cellulose furoates in 1-N-butyl-3-methylimidazolium chloride. Cellulose 14, 489–495 (2007). https://doi.org/10.1007/s10570-007-9138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-007-9138-8