Abstract
Paraquat poisoning results in significant pulmonary damage, but current treatments are only minimally effective in repairing the injured lung tissues. Recent research has highlighted the promise of using stem cell therapy, namely mesenchymal stem cells, as a new method for treating paraquat toxicity. These cells have shown effectiveness in decreasing inflammation, apoptosis, and fibrosis in the mice lungs subjected to paraquat. The therapeutic implications of mesenchymal stem cells are believed to arise from their release of bioactive proteins and their capacity to regulate inflammatory responses. However, additional clinical study is required to validate these therapies' efficacy. This review thoroughly explores the pathophysiology of paraquat poisoning and the properties of mesenchymal stem cells. Additionally, it critically assesses the long-term safety and effectiveness of mesenchymal stem cell therapies, which is crucial for developing more dependable and effective treatment protocols. In summary, although mesenchymal stem cells offer promising prospects for treating lung injuries, more investigations are required to optimize their therapeutic promise and ensure their safe clinical application in the context of paraquat poisoning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paraquat, a pyridine herbicide frequently employed in agricultural environments, is recognized for its substantial toxicity (Feng et al. 2018). Most paraquat poisoning cases result from intentional or unintentional ingestion, causing over 100,000 deaths each year and posing a significant health threat (Mew et al. 2017). Upon introduction into the human body via multiple pathways, such as dermal contact and ingestion, this compound tends to amass in the alveoli and bronchioles (Tang et al. 2024). This accumulation leads to direct harm to pulmonary tissues, causing notable lung damage and a variety of clinical presentations, including interstitial pneumonia, alveolitis, and pulmonary fibrosis (Geng et al. 2021). The efficacy of current therapeutic strategies for managing paraquat-induced lung injury is limited in their capacity to halt disease advancement (Li et al. 2023a). Consequently, there is a critical need to prioritize the development of innovative treatment modalities focused on paraquat-induced lung injury.
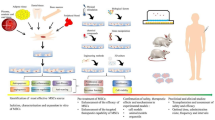
Recently, stem cell therapy gained more attention (Serna Villa and Ren 2024), particularly in the context of its potential therapeutic applications in various lung diseases (Long et al. 2024). Mesenchymal stem cells (MSC) have been the subject of extensive investigation in both preclinical and clinical research due to their favorable attributes, such as low immunogenicity and ease of acquisition (Shi et al. 2024). MSC has demonstrated suppressive implications that effectively mitigated inflammation, apoptosis, and fibrosis in paraquat-induced lung injury (He et al. 2018) (Fig. 1). This therapeutic outcome was attributed to the secretion of bioactive proteins and the regulation of inflammatory responses, ultimately leading to improved lung injury (Zhang et al. 2019a). Therefore, the use of MSC and its derivatives offers a promising new strategy for addressing paraquat-triggered lung damage.
The therapeutic effect of mesenchymal stem cells (MSC) therapy on paraquat-induced lung injury. HO1: heme oxygenase MDA: malondialdehyde; 1; GSH: glutathione; Nrf2: nuclear factor erythroid 2-related factor; SOD: superoxide dismutase; NF-κB: nuclear factor kappa-B; TLR4: toll-like receptor 4; SMA: smooth muscle actin; TNFα: tumor necrosis factor-alpha; IL: interleukin
This review examines the pathophysiological mechanisms of paraquat-triggered lung damage, evaluates the present advances in research regarding the efficacy of MSC therapy in treating paraquat-induced lung damage, and analyzes the impact of MSC-derived products on lung injury. The primary aim is to evaluate the viability of employing MSC therapy for treating paraquat-triggered lung injury.
Mechanism of paraquat-induced lung injury
Paraquat possesses a high-energy electron that interacts with mitochondria (Wang et al. 2024), extracting electrons and transferring them to oxygen molecules to generate reactive oxygen species (ROS) (Li et al. 2022a; Mahmoudi et al. 2023). ROS disrupts vital biomolecules and reduces levels of crucial antioxidant molecules such as glutathione, thereby compromising the cell's natural antioxidant defense system (Mirzaee et al. 2019). In addition, paraquat exposure causes oxidative stress, hindering nicotinamide adenine dinucleotide phosphate, resulting in increased levels of superoxide anions and hydrogen peroxide (Elkholy et al. 2023). The dismutation of hydrogen peroxide led to the generation of hydroxyl radicals, initiating a cascade of chain reactions, including lipid peroxidation. These processes ultimately led to the modification of cell membrane structure and function (Yadav and Singh 2022). Furthermore, paraquat-induced triggered the generation of nitric oxide by upregulating the nitric oxide synthase expression (Cui et al. 2023). Exposure to paraquat has been shown to disrupt the balance between oxidation and antioxidant defense in pulmonary cells (Li et al. 2022b; Song et al. 2023; Zhang et al. 2021a).
Paraquat has been shown to induce the activation of multiple cell types in the pulmonary system, leading to an immune-mediated inflammatory response (Wu et al. 2020; Jiang et al. 2022). Exposure to paraquat has been linked to an elevation in transforming growth factor (TGF)-β expression in macrophages (He et al. 2022). Additionally, paraquat promotes M1 polarization of alveolar macrophages through glycolysis, exacerbating inflammatory reactions and contributing to acute lung injury, as indicated by elevated levels of proinflammatory cytokines and glycolytic enzymes following paraquat exposure (Zhang et al. 2021b; Zhang et al. 2022a). Moreover, paraquat has been shown to activate the innate immune system, resulting in aberrantly activated natural killer (NK) cells that promote proinflammatory M1 macrophage polarization, thereby exacerbating inflammation and tissue damage (Wu et al. 2020). Paraquat exposure also upregulates metallothionein-1 expression, which in turn reduces free zinc ions necessary for NK cell function and GATA binding protein-3 expression, potentially leading to immunosuppression by impairing NK and T-cell maturation and activity (Lim et al. 2015). Additionally, paraquat-induced aggregation and stimulation of monocytes have been observed, with activated monocytes secreting chemotactic factors that contribute to the inflammatory response (Amirshahrokhi and Khalili 2016). Paraquat directly stimulated monocytes, producing the proinflammatory cytokine interleukin (IL)-8. Furthermore, it facilitated the aggregation and activation of neutrophils (Bianchi et al. 1993). Additional research has shown that paraquat triggers a signaling pathway involving high-mobility group box 1, Toll-like receptor 4, IL-23, and ultimately producing IL-17A and γδT cells (Yan et al. 2017). Moreover, paraquat was observed to disrupt the balance between T helper-17 and regulatory T cells (Yang et al. 2017). Patients with human immunodeficiency virus infection demonstrated more positive outcomes after paraquat poisoning compared to other patient groups, suggesting a potential role of CD4+T cells (Shang and Lu 2015; Lu 2018). Transplanting T lymphocytes into mice with congenital immune deficiencies exacerbated paraquat-induced pulmonary fibrosis (Hu et al. 2021). Overall, paraquat significantly impacts immune cells in the pulmonary system, affecting complex immune-inflammatory networks.
Paraquat induces pulmonary tissue damage through the initiation of various forms of cellular death, including epithelial to mesenchymal transition, leading to the development of pulmonary fibrosis (Liu et al. 2022a). This process is facilitated by the inhibition of the interaction between the autophagy-specific substrate sequestosome 1 (p62) and microtubule-associated protein 1 light chain 3 (LC3) in alveolar epithelial cells (Jiang et al. 2021). Additionally, Paraquat inhibited the extracellular regulated protein kinases (ERK) phosphorylation, impacting the transcription and expression of the autophagy-related gene ATG12, thereby promoting accelerated aging of lung cells (Huang et al. 2022). Low-dose paraquat was found to induce cell autophagy and increase nuclear factor erythroid 2-related factor (Nrf2) and nuclear factor kappa-B (NF-κB) p65 levels, with minimal impact on Kelch-like ECH-associated protein 1 (Keap1) (Yao et al. 2019). In contrast, high-dose paraquat was observed to inhibit protective autophagy and trigger cell apoptosis. Subsequent investigations revealed that high-dose paraquat upregulated Keap1 expression, thereby suppressing Nrf2 transcriptional activity and hindering the expression of autophagy-related genes (Yao et al. 2019). Additionally, paraquat was shown to induce autophagy in lung tissues by modulating microRNA (mir) -193a, influencing phosphatidylinositide 3 kinase (PI3K)- serine/threonine kinase proteins (Akt)- mammalian target of rapamycin (mTOR) signaling pathways and oxidative stress markers (Liu et al. 2019a). Paraquat-induced apoptosis was mediated via the mitochondrial-dependent pathway (Rashidipour et al. 2021; Liu et al. 2022b), leading to reduced expression of the forkhead box F1 and inhibition of its protective functions against apoptosis and oxidative stress (Zheng et al. 2020). Furthermore, paraquat-induced downregulation of mir-141 relieved its inhibitory effect on the downstream target gene histone deacetylase 6, ultimately activating the NF-κB p65 signaling pathway and promoting pulmonary fibrosis (Zheng et al. 2021). Paraquat has been shown to trigger pyroptosis by stimulating caspase 3, initiating the cleavage activation of gasdermin E, and compromising cell membrane integrity (Tang et al. 2023). Furthermore, recent studies have revealed that paraquat induces ferroptosis. These include disruption of mitochondrial homeostasis, promotion of autophagy, the nuclear receptor coactivator 4- ferritin heavy chain 1 axis activation, and modulation of the Nrf2 antioxidant pathway, resulting in lipid peroxidation, and consequent ferroptosis (Du et al. 2024; Cai et al. 2023). Paraquat induces lipid peroxidation by reducing polyunsaturated fatty acid-containing phospholipids due to acyl-CoA synthetase long-chain family member 4 deficiency (Tomitsuka et al. 2023). The ferroptosis inhibition by a ferroptosis inhibitor involves chelating iron and reducing Nrf2 to mitigate lipid peroxidation (Song et al. 2023). In conclusion, paraquat triggers various forms of lung cellular death, including autophagy, apoptosis, pyroptosis, and ferroptosis (Fig. 2), suggesting potential avenues for further investigation in the prevention and treatment of paraquat poisoning.
Paraquat-associated cell death. Akt: serine/threonine kinase proteins; PI3K: phosphatidylinositide 3-kinase; mTOR, mammalian target of rapamycin; ERK, extracellular regulated protein kinases; ATG12, autophagy-related 12; LC3, microtubule-associated proteins light chain 3; ACSL4, acyl-CoA synthetase long chain family member 4; Keap1, kelch-like ECH-associated protein 1; p62, sequestosome 1; GPX4, glutathione peroxidase 4; mir, microRNA; HDAC, histone deacetylase; IKK, inhibitor of kappa B kinase; KLF5, kruppel-like factor5; GSDME, gasdermin E
MSC therapy in paraquat poisoning treatment
MSCs have been effectively extracted and cultured from several tissues, encompassing bone marrow, cord blood, adipose tissue, and gingiva (Chen et al. 2024; Jafari et al. 2024; Hyun et al. 2024). MSCs interact with immune cells and the inflammatory microenvironment, which can hinder their ability to engraft and differentiate in sufficient quantities to replace damaged tissues effectively (Ma et al. 2022; Szűcs et al. 2023). Consequently, the therapeutic effectiveness of MSCs is predominantly owing to their immunomodulatory and antioxidant properties (Liu et al. 2016). MSCs have been manifested to influence immune regulatory cell populations, including T cells and dendritic cells, and facilitate the polarization of macrophages towards a desired phenotype (Liu et al. 2019b). Moreover, MSCs induced apoptosis in proinflammatory cells and decreased the influx of inflammatory cells, specifically CD3+ T lymphocytes (Watanabe et al. 2019; He et al. 2020). MSCs regulated the C–C Motif Chemokine Receptor 2 (CCR2) monocyte chemoattractant protein-1 axis to hinder T cell activation and facilitate the development of immunoregulatory CD4+ T cells (Cao et al. 2021). Additionally, MSCs were essential in recognizing danger signals and mitigating tissue injury at sites of damage or disease (Park et al. 2018). The immunomodulatory implications of MSCs may hold promise for treating paraquat-induced lung injury.
As summarized in Table 1, the efficacy of MSCs in treating lung damage triggered by paraquat was confirmed in animal models. Mechanisms of BMSCs may involve multiple aspects of anti-inflammatory, anti-apoptotic, mitochondrial protection, and modulation of cellular signaling pathways, thereby attenuating lung injury at the molecular and cellular levels. Using a combination of transcriptome sequencing and molecular experiments, Zhang et al. (Zhang et al. 2020) found that paraquat treatment resulted in the up-or down-regulation of a large number of genes that contributed to multiple biological processes, encompassing oxidative stress responses, apoptotic processes, immune responses, and inflammatory responses. In PQ-treated samples, significant alterations were observed in key signaling events of the NIK/NF-kappaB and IL-17 pathways. However, these changes were substantially mitigated following MSC pretreatment. This was evidenced by increased phosphorylation of IκBα and NF-kappaB, along with elevated levels of caspases 3/9 and Cyclin D1 proteins following paraquat treatment. However, these changes were significantly attenuated in samples treated with MSC transplantation.
BMSC treatment may alleviate inflammation by reducing the expression of the proinflammatory factors, encompassing TNF-α and IL-1β/-6/10. Additionally, it may decrease the α-SMA and vimentin expression, thereby reducing fibrosis (Chen et al. 2019). The injection of bone marrow-derived mesenchymal stem cells (BM-MSCs) resulted in a significant hindrance in malondialdehyde levels and an elevation in superoxide dismutase (SOD) and glutathione levels (Zhang et al. 2019a; Yang et al. 2013). MSCs exert an antioxidative effect by increasing the HO-1 and Nrf-2 protein expression in lung tissues. Additionally, they decrease the levels of TLR4, NF-kappaB p65, and activated caspase-3 proteins, which effectively attenuate lung injury (Zhang et al. 2019a; Yang et al. 2013; Wu et al. 2023). Despite the lack of successful engraftment following in vivo transplantation, BM-MSCs demonstrated promising effects by upregulating antioxidative gene expression, including heme oxygenase 1 (HO-1) and metallothionein 1a (Tsai et al. 2013). BM-MSCs also demonstrated protective effects by regulating apoptosis-related proteins, including caspase-3 and B-cell lymphoma-2-associated X, through the NF-κB and ERK signaling pathways (Zhang et al. 2019a; Zhang et al. 2020; Zhang et al. 2019b). In addition, BM-MSCs shield L2 cells from oxidative harm by potentially sequestering PQ within MSCs and stimulating the expression of HO-1 and metallothionein 1a (Tsai et al. 2013). Adipose-derived MSCs were found to reduce lactate and inflammatory factor levels in blood plasma. They also decreased the inflammatory CD3+ T cells infiltration and the expression of inflammatory cytokines, including TNF-α, IL-6, TGF-β1, and lactic acid levels (He et al. 2020). Amnion-derived MSCs have been manifested to protect against lung damage by modulating the NF-κB pathway, suppressing the release of inflammatory factors, reducing oxidative stress, enhancing alveolar capillary permeability, and actively participating in tissue repair processes (Wu et al. 2023). Similarly, umbilical cord-derived MSCs have been found to mitigate the release of inflammatory cytokines, including IL-8 and intercellular cell adhesion molecule-1, by upregulating the Nrf2/HO-1 pathway and inhibiting the apoptosis process involving NF-κB and caspase-3 (Zhang et al. 2018). umbilical cord derived-MSCs and dental pulp stem cells (DPSCs) reduce the expression of TGF-β-induced proinflammatory cytokines (TNF-α and IL-1β/6/8). At the same time, MSC treatment decreased the expression of TGF-β-induced fibrosis indicators (α-SMA and VIM) (Geng et al. 2021). In summary, MSCs hold significant promise for providing therapeutic benefits in cases of paraquat poisoning (Fig. 3).
Nevertheless, the therapeutic efficacy of MSCs from different sources shows slight variations. For instance, one study found that dental pulp-derived MSCs exhibited superior anti-inflammatory and anti-fibrotic properties compared to umbilical cord-derived MSCs (Geng et al. 2021). Additional investigation is warranted to ascertain the most suitable sources, timing, dosage, and route of administration for MSC injection to establish a safe and efficacious treatment regimen.
Strategies to optimize stem cell therapy
Currently, there is ongoing active clinical research on the use of mesenchymal stem cells (MSCs) for the treatment of lung injury, encompassing a range of lung diseases such as acute respiratory distress syndrome (ARDS), COVID-19 sequelae, mustard gas poisoning, and idiopathic pulmonary fibrosis. (ChiCTR2400081032, ChiCTR2300069181, NCT02749448, NCT02745184). However, challenges in the field of MSC therapy include considerations related to source selection, age-related concerns, replicative senescence, and blood compatibility, all of which are crucial in maximizing the therapeutic potential of MSCs (Kadri et al. 2023; Li et al. 2023b). Variations in biological morphology, proliferation capacity, differentiation potential, secretome composition, and immunomodulatory characteristics have been documented among MSCs extracted from different tissue sources, and these differences have been exhibited to impact the overall therapeutic effectiveness of MSC-based treatments directly (Sipp et al. 2018). The aging process significantly affects MSCs, as demonstrated by changes in cell ultrastructure in samples from older donors, leading to a notable decline in their ability to proliferate (Szűcs et al. 2023; Babenko et al. 2021). The in vitro cultivation of MSCs ultimately results in replicative senescence, a condition marked by genomic instability. As the number of cell replications increases, replicative senescence occurs, leading to the accumulation of cell damage during the aging process (Bakopoulou et al. 2017), ultimately impairing the functionality of MSCs. The secreted factors from senescent MSCs fail to support type 2 alveolar epithelial cells in their capacity to respond to different damages, thereby hindering their ability to reconstitute into alveolar spheroids (Chanda et al. 2021). The factors secreted by MSCs are insufficient to support type 2 alveolar epithelial cells in responding to various injuries, thereby impairing their ability to regenerate into alveolar spheroids (Moll et al. 2022; Moll et al. 2019). Additionally, concerns regarding their potential contribution to fibrosis cannot be overlooked. Studies utilizing genetic pedigree tracing techniques have identified MSC-like cells surrounding blood vessels as the primary source of organ fibrosis, indicating that these cells may be involved in the pathogenesis of fibrotic diseases rather than merely facilitating repair or preventing damage (Kramann et al. 2015). In liver injury models, transplantation of adult BM-MSCs has been observed to promote fibroblast formation (Baertschiger et al. 2009). Given the complexities associated with stem cell therapy, it is advisable to explore novel approaches, such as modified MSC therapy and extracellular vesicle therapy, for implementation.
Modified MSC therapy
Modified MSC therapy is a strategic method designed to increase the therapeutic efficacy of MSCs. The primary methods employed for optimization include gene modification and pre-activation (Fig. 4). Gene modification involves the overexpression of functional genes to enhance the capabilities of MSCs. For example, MSCs transfected with genes such as TGF-β, SOD2, or HGF have shown enhanced therapeutic outcomes in various treatments (Geng et al. 2021; Zhang et al. 2018; Xue et al. 2013). Transfection of the decorin gene in mesenchymal stem cells (MSCs) has been shown to inhibit the TGF-β1-Smad3 signaling pathway, leading to a reduction in inflammatory response and lung tissue fibrosis, ultimately improving lung function (Xu et al. 2024). Pre-activation involves recreating complex ex vivo environments in vivo to support MSCs and preserve their biological properties. These pre-activation conditions include factors such as hypoxia, 3D culture, cytokines, and bioactive compounds. Hypoxia-preactivated MSCs have shown enhanced therapeutic efficacy in treating lung injury compared to non-preactivated MSCs (Wang et al. 2021). The induction of a hypoxic environment in MSCs elicited a response from hypoxia-inducible factor α, leading to a rise in secreting hepatocyte growth factor and prolonged in vivo retention of MSCs (Lan et al. 2015; Hao et al. 2022). It was noted that under hypoxic circumstances, MSCs could decline lung inflammation by inducing the transformation of anti-inflammatory macrophages via paracrine signaling (Xu et al. 2022). MSC scaffolds enhance angiogenic and anti-inflammatory gene expression, reduce apoptosis and fibrosis gene expression, and promote survival, proliferation, and migration of alveolar type II cells in injured lungs. They also stimulate the regeneration of alveolar type I cells (Kudinov et al. 2022). MSCs can be utilized to bind to liposomes containing nintedanib, leveraging their homing properties to target drug delivery specifically to fibrotic lung tissue, thereby promoting repair and regeneration through collagen degradation and inhibition of fibroblast activation (Han et al. 2023). Additionally, the use of a dextran derivative as a ROS scavenger and CT imaging tracer has been demonstrated to enhance the survival rate of MSCs in the treatment of idiopathic pulmonary fibrosis by eliminating excess ROS (Lv et al. 2023). MSCs combined with inflammatory cell factors, either individually or in combination, provide a novel approach to enhance the paracrine and immunomodulatory functions of MSCs, thereby addressing their limited effectiveness (Chen et al. 2023; Su et al. 2015). The addition of acetate has been demonstrated to mitigate epigenetic changes in senescent MSCs (Pouikli et al. 2021). Furthermore, the suppressive effects of Chinese medicine and hydroxybenzene on the TLR4/P65 signaling pathway were markedly potentiated (He et al. 2021a; Zhang et al. 2022b). The utilization of autologous transplantation of mitochondria in MSCs has been suggested as a feasible approach to prolong the lifespan of MSC implantation (Yao et al. 2022; Zorova et al. 2022). These optimization techniques hold significant promise in the management of conditions such as lung damage and pulmonary fibrosis.
Specifically, in cases of paraquat-triggered lung damage, the use of HGF-modified dental pulp stem cells has demonstrated efficacy in mitigating inflammatory and fibrotic reactions (Geng et al. 2021). Nitric oxide-mediated induced pluripotent stem cells exhibit enhanced abilities for proliferation and migration (Cui et al. 2023). By upregulating the expression of HO-1 and Nrf-2 proteins and increasing the activities of SOD and glutathione peroxidase in lung tissue, this approach effectively inhibits the inflammatory response, enhances pulmonary microvascular permeability, and mitigates lung inflammation and damage induced by paraquat. Modifying stem cells to enhance their therapeutic capabilities represents a new method for treating paraquat-induced lung injury.
Extracellular particles derived from MSCs
MSC-derived extracellular particles (MSC-EPs) are characterized as bilayer lipid vesicles released by MSCs, serving a pivotal function in mediating intercellular communication and material exchange (Welsh et al. 2024). MSC-EPs present potential advantages over traditional MSC transplantation (Carter et al. 2019), particularly in the realm of targeted therapy, achieved through the delivery of bioactive molecules encompassing hepatocyte growth factor and miRNA to specific cellular and tissue targets (Chen et al. 2020). Furthermore, investigations into the involvement of mitochondria in MSC-EPs have garnered significant interest. In lung injury, the mitochondria transfer from MSC-EPs to lung epithelial cells has been observed to mitigate oxidative stress-induced damage (Islam et al. 2012; Zhao et al. 2021). This transfer has also been shown to attenuate damage to the lung epithelial cells. Alveolar macrophages that were exposed to mitochondria derived from MSC-EVs demonstrated increased levels of oxidative phosphorylation, which enhanced their anti-inflammatory and phagocytic capacities, ultimately resulting in reduced lung injury (Xia et al. 2022). Furthermore, MSC-EPs have been found to enhance mitochondrial function and respiration by integrating mitochondria into pre-existing mitochondrial networks (Dutra Silva et al. 2021). Apoptotic bodies originating from MSCs were observed to induce the activation of myeloid-epithelial-reproductive tyrosine kinase, leading to the stimulation of IL-10-producing neutrophil production, inhibition of NK cell activation, and promotion of macrophage polarization towards the M2 phenotype (He et al. 2021b).
EPs effectively transport therapeutic cargo to specific target cells, enabling their utility as efficient drug delivery vehicles (Song et al. 2024). EPs derived from various sources have demonstrated promising therapeutic outcomes in the treatment of lung injury. Notably, platelet-derived EPs loaded with TPCA-1, an inhibitor of the NF-κB pathway, have demonstrated targeted delivery to the lungs, effectively inhibiting inflammation (Ma et al. 2020). Engineered EPs containing IL-4/10 demonstrated a significant decrease in the secretion of proinflammatory cytokines, neutrophil infiltration, and lung injury (Salazar-Puerta et al. 2023). Although there is limited research on the potential of MSC-derived EPs to enhance the treatment of paraquat-induced lung injury, they show promising therapeutic benefits as a drug delivery system due to their low immunogenicity and high permeability (Feng et al. 2024). In paraquat, the DPSC-EPs implication on reducing the expression of fibrotic factors was better than that of the DPSC supernatant (Geng et al. 2021).
Conclusion
MSCs mitigated paraquat-induced lung injury primarily through secreting paracrine factors and modulation of the immune system (Fig. 5). This regulatory function serves to attenuate excessive inflammation and alleviate the extent of pulmonary fibrosis. It is pertinent to acknowledge the current lack of conclusive evidence regarding the in vivo differentiation and replacement of damaged tissue by MSCs. Furthermore, it is imperative to meticulously choose the suitable origin of MSCs and manage replicative aging when contemplating the utilization of MSCs. Subsequent research endeavors should delve deeper into comprehending the safety, effectiveness, and mechanism of MSCs in paraquat-induced lung injury and persist in optimizing therapeutic approaches. In summary, MSCs provide a hopeful strategy for addressing lung injury.
Data availability
No datasets were generated or analysed during the current study.
References
Amirshahrokhi K, Khalili A-R. Carvedilol attenuates paraquat-induced lung injury by inhibition of proinflammatory cytokines, chemokine MCP-1, NF-κB activation and oxidative stress mediators. Cytokine. 2016;88:144–53. https://doi.org/10.1016/j.cyto.2016.09.004.
Babenko VA, Silachev DN, Danilina TI, et al. Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells. Cells. 2021;10(6) https://doi.org/10.3390/cells10061273.
Baertschiger RM, Serre-Beinier Vr, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4(8):e6657. https://doi.org/10.1371/journal.pone.0006657.
Bakopoulou A, Apatzidou D, Aggelidou E, et al. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res Ther. 2017;8(1):247. https://doi.org/10.1186/s13287-017-0705-0.
Bianchi M, Fantuzzi G, Bertini R, Perin L, Salmona M, Ghezzi P. The pneumotoxicant paraquat induces IL-8 mRNA in human mononuclear cells and pulmonary epithelial cells. Cytokine. 1993;5(5):525–30.
Cai Q, Shen Q, Zhu W, Zhang S, Ke J, Lu Z. Paraquat-induced ferroptosis suppression via NRF2 expression regulation. Toxicol in Vitro. 2023;92: 105655. https://doi.org/10.1016/j.tiv.2023.105655.
Cao M, Liu H, Dong Y, et al. Mesenchymal stem cells alleviate idiopathic pneumonia syndrome by modulating T cell function through CCR2-CCL2 axis. Stem Cell Res Ther. 2021;12(1):378. https://doi.org/10.1186/s13287-021-02459-7.
Carter K, Lee HJ, Na K-S, et al. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019;99:247–57. https://doi.org/10.1016/j.actbio.2019.09.022.
Chanda D, Rehan M, Smith SR, et al. Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells. Elife. 2021;10https://doi.org/10.7554/eLife.68049.
Chen J, Si L, Zhou L, Deng Y. Role of bone marrow mesenchymal stem cells in the development of PQ-induced pulmonary fibrosis. Mol Med Rep. 2019;19(4):3283–90. https://doi.org/10.3892/mmr.2019.9976.
Chen W-X, Zhou J, Zhou S-S, et al. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Res Ther. 2020;11(1):113. https://doi.org/10.1186/s13287-020-01617-7.
Chen Z, Yao M-W, Shen Z-L, et al. Interferon-gamma and tumor necrosis factor-alpha synergistically enhance the immunosuppressive capacity of human umbilical-cord-derived mesenchymal stem cells by increasing PD-L1 expression. World J Stem Cells. 2023;15(8):787–806. https://doi.org/10.4252/wjsc.v15.i8.787.
Chen Y, Yang X, Feng M, Yu Y, Hu Y, Jiang W. Exosomal miR-223-3p from bone marrow mesenchymal stem cells targets HDAC2 to downregulate STAT3 phosphorylation to alleviate HBx-induced ferroptosis in podocytes. Front Pharmacol. 2024;15:1327149. https://doi.org/10.3389/fphar.2024.1327149.
Cui A, Li S, Li Y, et al. Nitric oxide-mediated the therapeutic properties of induced pluripotent stem cell for paraquat-induced acute lung injury. Front Immunol. 2023;14:1136290. https://doi.org/10.3389/fimmu.2023.1136290.
Du J, Yu L, Yang X, et al. Regulation of NCOA4-mediated iron recycling ameliorates paraquat-induced lung injury by inhibiting ferroptosis. Cell Commun Signal. 2024;22(1):146. https://doi.org/10.1186/s12964-024-01520-1.
Dutra Silva J, Su Y, Calfee CS, et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58(1)https://doi.org/10.1183/13993003.02978-2020.
Elkholy AR, El-Sheakh AR, Suddek GM. Nilotinib alleviates paraquat-induced hepatic and pulmonary injury in rats via the Nrf2/Nf-kB axis. Int Immunopharmacol. 2023;124(Pt A): 110886. https://doi.org/10.1016/j.intimp.2023.110886.
Feng M-X, Li Y-N, Ruan W-S, Lu Y-Q. Predictive value of the maximum serum creatinine value and growth rate in acute paraquat poisoning patients. Sci Rep. 2018;8(1):11587. https://doi.org/10.1038/s41598-018-29800-0.
Feng Y, Guo K, Jiang J, Lin S. Mesenchymal stem cell-derived exosomes as delivery vehicles for non-coding RNAs in lung diseases. Biomed Pharmacother. 2024;170: 116008. https://doi.org/10.1016/j.biopha.2023.116008.
Geng P, Zhang Y, Zhang H, et al. HGF-Modified Dental Pulp Stem Cells Mitigate the Inflammatory and Fibrotic Responses in Paraquat-Induced Acute Respiratory Distress Syndrome. Stem Cells Int. 2021;2021:6662831. https://doi.org/10.1155/2021/6662831.
Han M-M, He X-Y, Tang L, et al. Nanoengineered mesenchymal stem cell therapy for pulmonary fibrosis in young and aged mice. Sci Adv. 2023;9(29):eadg5358. https://doi.org/10.1126/sciadv.adg5358.
Hao C, You J, Qiu H, et al. Hypoxic preconditioning improves the survival and pro-angiogenic capacity of transplanted human umbilical cord mesenchymal stem cells via HIF-1α signaling in a rat model of bronchopulmonary dysplasia. Biochem Biophys Res Commun. 2022;605:111–8. https://doi.org/10.1016/j.bbrc.2022.03.044.
He F, Zhou A, Feng S, Li Y, Liu T. Mesenchymal stem cell therapy for paraquat poisoning: A systematic review and meta-analysis of preclinical studies. PLoS ONE. 2018;13(3): e0194748. https://doi.org/10.1371/journal.pone.0194748.
He F, Wang Y, Li Y, Yu L. Human amniotic mesenchymal stem cells alleviate paraquat-induced pulmonary fibrosis in rats by inhibiting the inflammatory response. Life Sci. 2020;243: 117290. https://doi.org/10.1016/j.lfs.2020.117290.
He H, Yang T, Li F, Zhang L, Ling X. A novel study on the immunomodulatory effect of umbilical cord derived mesenchymal stem cells pretreated with traditional Chinese medicine Asarinin. Int Immunopharmacol. 2021a;100: 108054. https://doi.org/10.1016/j.intimp.2021.108054.
He X, Hong W, Yang J, et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Signal Transduct Target Ther. 2021b;6(1):270. https://doi.org/10.1038/s41392-021-00688-z.
He Q, Zhang W, Zhang J, Deng Y. Cannabinoid Analogue WIN 55212–2 Protects Paraquat-Induced Lung Injury and Enhances Macrophage M2 Polarization. Inflammation. 2022;45(6):2256–67. https://doi.org/10.1007/s10753-022-01688-z.
Hu Y, Qian C, Sun H, et al. Differences in epithelial-mesenchymal-transition in paraquat-induced pulmonary fibrosis in BALB/C and BALB/C (nu/nu) nude mice. Biomed Pharmacother. 2021;143: 112153. https://doi.org/10.1016/j.biopha.2021.112153.
Huang C, Xue X, Gong N, Jiang J. Ginsenoside Rg1 suppresses paraquat-induced epithelial cell senescence by enhancing autophagy in an ATG12-dependent manner. Environ Toxicol. 2022;37(9):2302–13. https://doi.org/10.1002/tox.23597.
Hyun J, Eom J, Im J, et al. Fibroblast function recovery through rejuvenation effect of nanovesicles extracted from human adipose-derived stem cells irradiated with red light. J Control Release. 2024. https://doi.org/10.1016/j.jconrel.2024.02.047.
Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65. https://doi.org/10.1038/nm.2736.
Jafari A, Vatanpour M, Barikrow N, Razavi P, Tour SS. Effect of erbium yttrium aluminium garnet laser dentin conditioning on dental pulp stem cells viability. Heliyon. 2024;10(5): e26954. https://doi.org/10.1016/j.heliyon.2024.e26954.
Jiang S, Zhang W, Lu Y. Development and validation of novel inflammatory response-related gene signature for sepsis prognosis. J Zhejiang Univ Sci B. 2022;23(12):1028–41. https://doi.org/10.1631/jzus.B2200285.
Jiang F, Li S, Jiang Y, Chen Z, Wang T, Liu W. Fluorofenidone attenuates paraquat‑induced pulmonary fibrosis by regulating the PI3K/Akt/mTOR signaling pathway and autophagy. Mol Med Rep. 2021;23(6)https://doi.org/10.3892/mmr.2021.12044.
Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol. 2023;20(6):613–25. https://doi.org/10.1038/s41423-023-01022-z.
Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. https://doi.org/10.1016/j.stem.2014.11.004.
Kudinov VA, Artyushev RI, Zurina IM, et al. Inhaled Placental Mesenchymal Stromal Cell Secretome from Two- and Three-Dimensional Cell Cultures Promotes Survival and Regeneration in Acute Lung Injury Model in Mice. Int J Mol Sci. 2022;23(7)https://doi.org/10.3390/ijms23073417.
Lan Y-W, Choo K-B, Chen C-M, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6(1):97. https://doi.org/10.1186/s13287-015-0081-6.
Li S, Zhou X, Zeng R, et al. YAP1 silencing attenuated lung injury/fibrosis but worsened diaphragmatic function by regulating oxidative stress and inflammation response in mice. Free Radic Biol Med. 2022a;193(Pt 2):485–98. https://doi.org/10.1016/j.freeradbiomed.2022.10.323.
Li W, Li M, Chen K, et al. Oxaloacetate acid ameliorates paraquat-induced acute lung injury by alleviating oxidative stress and mitochondrial dysfunction. Front Pharmacol. 2022b;13:1029775. https://doi.org/10.3389/fphar.2022.1029775.
Li C, Cai H, Meng F, et al. Case report: Lung transplantation for treatment of paraquat intoxication: timing of transplantation. Front Pharmacol. 2023a;14:1205689. https://doi.org/10.3389/fphar.2023.1205689.
Li T-T, Zhang B, Fang H, et al. Human mesenchymal stem cell therapy in severe COVID-19 patients: 2-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2023b;92: 104600. https://doi.org/10.1016/j.ebiom.2023.104600.
Lim JH, Won JH, Ahn KH, et al. Paraquat reduces natural killer cell activity via metallothionein induction. J Immunotoxicol. 2015;12(4):342–9. https://doi.org/10.3109/1547691X.2014.980924.
Liu J, Kuwabara A, Kamio Y, et al. Human Mesenchymal Stem Cell-Derived Microvesicles Prevent the Rupture of Intracranial Aneurysm in Part by Suppression of Mast Cell Activation via a PGE2-Dependent Mechanism. Stem Cells. 2016;34(12):2943–55. https://doi.org/10.1002/stem.2448.
Liu M-W, Su M-X, Tang D-Y, Hao L, Xun X-H, Huang Y-Q. Ligustrazin increases lung cell autophagy and ameliorates paraquat-induced pulmonary fibrosis by inhibiting PI3K/Akt/mTOR and hedgehog signalling via increasing miR-193a expression. BMC Pulm Med. 2019a;19(1):35. https://doi.org/10.1186/s12890-019-0799-5.
Liu J, Feng B, Xu Y, et al. Immunomodulatory effect of mesenchymal stem cells in chemical-induced liver injury: a high-dimensional analysis. Stem Cell Res Ther. 2019b;10(1):262. https://doi.org/10.1186/s13287-019-1379-6.
Liu X, Yang H, Liu Z. Signaling pathways involved in paraquat-induced pulmonary toxicity: Molecular mechanisms and potential therapeutic drugs. Int Immunopharmacol. 2022a;113(Pt A): 109301. https://doi.org/10.1016/j.intimp.2022.109301.
Liu C, Sun Z, Wang M, et al. Mitoquinone mitigates paraquat-induced A549 lung epithelial cell injury by promoting MFN1/MFN2-mediated mitochondrial fusion. J Biochem Mol Toxicol. 2022b;36(9): e23127. https://doi.org/10.1002/jbt.23127.
Long Y, Yang B, Lei Q, et al. Targeting Senescent Alveolar Epithelial Cells Using Engineered Mesenchymal Stem Cell-Derived Extracellular Vesicles To Treat Pulmonary Fibrosis. ACS Nano. 2024;18(9):7046–63. https://doi.org/10.1021/acsnano.3c10547.
Lu Y-Q. HIV and paraquat poisoning: fighting fire with fire? J Zhejiang Univ Sci B. 2018;19(2):168–70.
Lv Y, Yu C, Li X, et al. ROS-activatable nanocomposites for CT imaging tracking and antioxidative protection of mesenchymal stem cells in idiopathic pulmonary fibrosis therapy. J Control Release. 2023;357:249–63. https://doi.org/10.1016/j.jconrel.2023.03.057.
Ma Q, Fan Q, Xu J, et al. Calming Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter. 2020;3(1):287–301. https://doi.org/10.1016/j.matt.2020.05.017.
Ma C, Han L, Wu J, et al. MSCs cell fates in murine acute liver injury and chronic liver fibrosis induced by carbon tetrachloride. Drug Metab Dispos. 2022. https://doi.org/10.1124/dmd.122.000958.
Mahmoudi Z, Kalantar H, Mansouri E, Mohammadi E, Khodayar MJ. Dimethyl fumarate attenuates paraquat-induced pulmonary oxidative stress, inflammation and fibrosis in mice. Pestic Biochem Physiol. 2023;190: 105336. https://doi.org/10.1016/j.pestbp.2023.105336.
Mew EJ, Padmanathan P, Konradsen F, et al. The global burden of fatal self-poisoning with pesticides 2006–15: Systematic review. J Affect Disord. 2017;219https://doi.org/10.1016/j.jad.2017.05.002.
Mirzaee S, Mansouri E, Shirani M, Zeinvand-Lorestani M, Khodayar MJ. Diosmin ameliorative effects on oxidative stress and fibrosis in paraquat-induced lung injury in mice. Environ Sci Pollut Res Int. 2019;26(36):36468–77. https://doi.org/10.1007/s11356-019-06572-2.
Moll G, Ankrum JA, Kamhieh-Milz J, et al. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med. 2019;25(2):149–63. https://doi.org/10.1016/j.molmed.2018.12.006.
Moll G, Ankrum JA, Olson SD, Nolta JA. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Transl Med. 2022;11(1)https://doi.org/10.1093/stcltm/szab005.
Park S-R, Kim H-J, Yang S-R, Park CH, Lee H-Y, Hong I-S. A novel endogenous damage signal, glycyl tRNA synthetase, activates multiple beneficial functions of mesenchymal stem cells. Cell Death Differ. 2018;25(11):2023–36. https://doi.org/10.1038/s41418-018-0099-2.
Pouikli A, Parekh S, Maleszewska M, et al. Chromatin remodeling due to degradation of citrate carrier impairs osteogenesis of aged mesenchymal stem cells. Nat Aging. 2021;1(9):810–25. https://doi.org/10.1038/s43587-021-00105-8.
Rashidipour M, Rasoulian B, Maleki A, Davari B, Pajouhi N, Mohammadi E. Pectin/chitosan/tripolyphosphate encapsulation protects the rat lung from fibrosis and apoptosis induced by paraquat inhalation. Pestic Biochem Physiol. 2021;178: 104919. https://doi.org/10.1016/j.pestbp.2021.104919.
Salazar-Puerta AI, Rincon-Benavides MaA, Cuellar-Gaviria TZ, et al. Engineered Extracellular Vesicles Derived from Dermal Fibroblasts Attenuate Inflammation in a Murine Model of Acute Lung Injury. Adv Mater. 2023;35(28):e2210579. https://doi.org/10.1002/adma.202210579.
Serna Villa V, Ren X. Lung Progenitor and Stem Cell Transplantation as a Potential Regenerative Therapy for Lung Diseases. Transplantation. 2024. https://doi.org/10.1097/TP.0000000000004959.
Shang A-D, Lu Y-Q. A case report of severe paraquat poisoning in an HIV-positive patient: an unexpected outcome and inspiration. Medicine (baltimore). 2015;94(8): e587. https://doi.org/10.1097/MD.0000000000000587.
Shi H, Yang Z, Cui J, Tao H, Ma R, Zhao Y. Mesenchymal stem cell-derived exosomes: a promising alternative in the therapy of preeclampsia. Stem Cell Res Ther. 2024;15(1):30. https://doi.org/10.1186/s13287-024-03652-0.
Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561(7724):455–7. https://doi.org/10.1038/d41586-018-06756-9.
Song Y, Hu J, Ma C, Liu H, Li Z, Yang Y. Macrophage-Derived Exosomes as Advanced Therapeutics for Inflammation: Current Progress and Future Perspectives. Int J Nanomedicine. 2024;19:1597–627. https://doi.org/10.2147/IJN.S449388.
Song C-Y, Feng M-X, Li L, Wang P, Lu X, Lu Y-Q. Tripterygium wilfordii Hook.f. ameliorates paraquat-induced lung injury by reducing oxidative stress and ferroptosis via Nrf2/HO-1 pathway. Ecotoxicol Environ Saf. 2023;252:114575. https://doi.org/10.1016/j.ecoenv.2023.114575.
Su W, Wan Q, Huang J, et al. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136(2)https://doi.org/10.1016/j.jaci.2014.12.1926.
Szűcs D, Miklós V, Monostori T, et al. Effect of inflammatory microenvironment on the regenerative capacity of adipose-derived mesenchymal stem cells. Cells. 2023;12(15):1966. https://doi.org/10.3390/cells12151966.
Tang Y, Chen K, Xiao Z, et al. A novel mechanism of Dimethyl ester of Alpha-ketoglutarate in suppressing Paraquat-induced BEAS-2B cell injury by alleviating GSDME dependent pyroptosis. Phytomedicine. 2023;112: 154698. https://doi.org/10.1016/j.phymed.2023.154698.
Tang G, Jiang Z, Xu L, Yang Y, Yang S, Yao R. Development and validation of a prognostic nomogram for predicting in-hospital mortality of patients with acute paraquat poisoning. Sci Rep. 2024;14(1):1622. https://doi.org/10.1038/s41598-023-50722-z.
Tomitsuka Y, Imaeda H, Ito H, et al. Gene deletion of long-chain acyl-CoA synthetase 4 attenuates xenobiotic chemical-induced lung injury via the suppression of lipid peroxidation. Redox Biol. 2023;66: 102850. https://doi.org/10.1016/j.redox.2023.102850.
Tsai H-L, Chang J-W, Yang H-W, et al. Amelioration of paraquat-induced pulmonary injury by mesenchymal stem cells. Cell Transplant. 2013;22(9):1667–81. https://doi.org/10.3727/096368912X657765.
Wang Y, Li H, Li X, Su X, Xiao H, Yang J. Hypoxic Preconditioning of Human Umbilical Cord Mesenchymal Stem Cells Is an Effective Strategy for Treating Acute Lung Injury. Stem Cells Dev. 2021;30(3):128–34. https://doi.org/10.1089/scd.2020.0174.
Wang Q, Wang L, Huang Z, et al. Abalone peptide increases stress resilience and cost-free longevity via SKN-1-governed transcriptional metabolic reprogramming in C. elegans. Aging Cell. 2024;23(2):e14046. https://doi.org/10.1111/acel.14046.
Watanabe Y, Tsuchiya A, Seino S, et al. Mesenchymal Stem Cells and Induced Bone Marrow-Derived Macrophages Synergistically Improve Liver Fibrosis in Mice. Stem Cells Transl Med. 2019;8(3):271–84. https://doi.org/10.1002/sctm.18-0105.
Welsh JA, Goberdhan DCI, O’Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13(2): e12404. https://doi.org/10.1002/jev2.12404.
Wu M, Zhou C, Li M, et al. Depletion of NK cells attenuates paraquat-induced acute lung injury by manipulating macrophage polarization. Int Immunopharmacol. 2020;86: 106698. https://doi.org/10.1016/j.intimp.2020.106698.
Wu Y, Sun H, Qin L, et al. Human amnion-derived mesenchymal stem cells attenuate acute lung injury in two different acute lung injury mice models. Front Pharmacol. 2023;14:1149659. https://doi.org/10.3389/fphar.2023.1149659.
Xia L, Zhang C, Lv N, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12(6):2928–47. https://doi.org/10.7150/thno.69533.
Xu W, Li CK, Yang LS, Nasab EM, Athari SS, Gu WD. Immune response regulation by transduced mesenchymal stem cells with decorin gene on bleomycin-induced lung injury, fibrosis, and inflammation. Allergol Immunopathol (madr). 2024;52(4):53–9. https://doi.org/10.15586/aei.v52i4.1104.
Xu Z, Lin L, Fan Y, et al. Secretome of Mesenchymal Stem Cells from Consecutive Hypoxic Cultures Promotes Resolution of Lung Inflammation by Reprogramming Anti-Inflammatory Macrophages. Int J Mol Sci. 2022;23(8)https://doi.org/10.3390/ijms23084333.
Xue J, Li X, Lu Y, et al. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol Ther. 2013;21(2):456–65. https://doi.org/10.1038/mt.2012.183.
Yadav A, Singh C. Cyclooxygenase-2 activates the free radical-mediated apoptosis of polymorphonuclear leukocytes in the maneb- and paraquat-intoxicated rats. Pestic Biochem Physiol. 2022;187: 105202. https://doi.org/10.1016/j.pestbp.2022.105202.
Yan B, Chen F, Xu L, Xing J, Wang X. HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung injury by mediating neutrophil infiltration in mice. Sci Rep. 2017;7(1):597. https://doi.org/10.1038/s41598-017-00721-8.
Yang H, Wen Y, Bin J, Hou-You Y, Yu-Tong W. Protection of bone marrow mesenchymal stem cells from acute lung injury induced by paraquat poisoning. Clin Toxicol (phila). 2011;49(4):298–302. https://doi.org/10.3109/15563650.2011.566882.
Yang X, Zhang J-H, Zhang J-F, et al. Imbalance of Th17/Treg in the Pathogenesis of Mice with Paraquat-induced Acute Lung Injury. Iran J Allergy Asthma Immunol. 2017;16(6):511–9.
Yang H, Wen Y, Hou-you Y, et al. Combined treatment with bone marrow mesenchymal stem cells and methylprednisolone in paraquat-induced acute lung injury. BMC Emerg Med. 2013;13 Suppl 1(Suppl 1):S5. https://doi.org/10.1186/1471-227X-13-S1-S5.
Yao J, Zhang J, Tai W, et al. High-Dose Paraquat Induces Human Bronchial 16HBE Cell Death and Aggravates Acute Lung Intoxication in Mice by Regulating Keap1/p65/Nrf2 Signal Pathway. Inflammation. 2019;42(2):471–84. https://doi.org/10.1007/s10753-018-00956-1.
Yao X, Ma Y, Zhou W, et al. In-cytoplasm mitochondrial transplantation for mesenchymal stem cells engineering and tissue regeneration. Bioeng Transl Med. 2022;7(1): e10250. https://doi.org/10.1002/btm2.10250.
Zhang L-C, Wang Y, Liu W, Zhang X-M, Fan M, Zhao M. Protective effects of SOD2 overexpression in human umbilical cord mesenchymal stem cells on lung injury induced by acute paraquat poisoning in rats. Life Sci. 2018;214:11–21. https://doi.org/10.1016/j.lfs.2018.10.020.
Zhang L, Li Q, Liu W, Liu Z, Shen H, Zhao M. Mesenchymal Stem Cells Alleviate Acute Lung Injury and Inflammatory Responses Induced by Paraquat Poisoning. Med Sci Monit. 2019a;25:2623–32. https://doi.org/10.12659/MSM.915804.
Zhang L, Li Q, Liu Z, Wang Y, Zhao M. The protective effects of bone mesenchymal stem cells on paraquat-induced acute lung injury via the muc5b and ERK/MAPK signaling pathways. Am J Transl Res. 2019b;11(6):3707–21.
Zhang L, Wang Y, Shen H, Zhao M. Combined signaling of NF-kappaB and IL-17 contributes to Mesenchymal stem cells-mediated protection for Paraquat-induced acute lung injury. BMC Pulm Med. 2020;20(1):195. https://doi.org/10.1186/s12890-020-01232-5.
Zhang Z-D, Yang Y-J, Liu X-W, Qin Z, Li S-H, Li J-Y. Aspirin eugenol ester ameliorates paraquat-induced oxidative damage through ROS/p38-MAPK-mediated mitochondrial apoptosis pathway. Toxicology. 2021a;453: 152721. https://doi.org/10.1016/j.tox.2021.152721.
Zhang Y, Yuan D, Li Y, et al. Paraquat promotes acute lung injury in rats by regulating alveolar macrophage polarization through glycolysis. Ecotoxicol Environ Saf. 2021b;223: 112571. https://doi.org/10.1016/j.ecoenv.2021.112571.
Zhang Q, Wang Y, Wang Z, et al. Synthesis and anti-inflammatory activities of glycyrrhetinic acid derivatives containing disulfide bond. Bioorg Chem. 2022a;119: 105542. https://doi.org/10.1016/j.bioorg.2021.105542.
Zhang Y, Yang S, Qiu Z, et al. Pyrogallol enhances therapeutic effect of human umbilical cord mesenchymal stem cells against LPS-mediated inflammation and lung injury via activation of Nrf2/HO-1 signaling. Free Radic Biol Med. 2022b;191:66–81. https://doi.org/10.1016/j.freeradbiomed.2022.08.030.
Zhao M, Liu S, Wang C, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano. 2021;15(1):1519–38. https://doi.org/10.1021/acsnano.0c08947.
Zheng F, Liu T, Zhu J, Xie Y, Wu L, Lin Z. FoxF1 protects rats from paraquat-evoked lung injury following HDAC2 inhibition via the microRNA-342/KLF5/IκB/NF-κB p65 axis. Exp Cell Res. 2020;395(2): 112208. https://doi.org/10.1016/j.yexcr.2020.112208.
Zheng F, Zhu J, Zhang W, Fu Y, Lin Z. Thal protects against paraquat-induced lung injury through a microRNA-141/HDAC6/IκBα-NF-κB axis in rat and cell models. Basic Clin Pharmacol Toxicol. 2021;128(2):334–47. https://doi.org/10.1111/bcpt.13505.
Zorova LD, Kovalchuk SI, Popkov VA, et al. Do Extracellular Vesicles Derived from Mesenchymal Stem Cells Contain Functional Mitochondria? Int J Mol Sci. 2022;23(13)https://doi.org/10.3390/ijms23137408.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFC3603100 and 2023YFC3603105).
Funding
This work was supported by the National Key Research and Development Program of China (2023YFC3603100 and 2023YFC3603105).
Author information
Authors and Affiliations
Contributions
They wrote the main manuscript text and prepared the figures. Yuan-Qiang Lu, the corresponding author, conceptualized the study, supervised the project, and critically revised the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethical approval
As this review article is based on previously published studies and does not involve any new data collection from human or animal subjects, ethical approval was not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Li, T. & Lu, YQ. Mesenchymal stem cell-based therapy for paraquat-induced lung injury. Cell Biol Toxicol 40, 70 (2024). https://doi.org/10.1007/s10565-024-09911-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10565-024-09911-3