Abstract
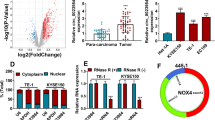
Investigation on a competitive endogenous RNA (ceRNA) network attracted lots of attention due its function in cancer regulation. Here, we probed into the possible molecular mechanism of circSSPO/microRNA-6820-5p (miR-6820-5p)/kallikrein-related peptidase 8 (KLK8)/PKD1 network in the esophageal squamous cell carcinoma (ESCC). Following whole-transcriptome sequencing and differential analysis in collected ESCC tissue samples, circRNA-miRNA-mRNA regulatory network affecting ESCC was investigated. After interaction measurement among circSSPO/miR-6820-5p/KLK8/PKD1, their regulatory roles in ESCC cell functions in vitro and xenograft tumor growth and lung metastasis in vivo were analyzed. The bioinformatics prediction and sequencing results screened that circSSPO, miR-6820-5p, KLK8, and PKD1 were associated with ESCC development. In ESCC, miR-6820-5p was expressed at very low levels, while circSSPO, KLK8, and PKD1 were highly expressed. In vitro cell experiments further proved that circSSPO competitively inhibited miR-6820-5p to induce ESCC cell malignant properties. Moreover, knockdown of KLK8 or PKD1 inhibited ESCC cell malignant properties. circSSPO also promoted the tumorigenic and metastasis of ESCC through the upregulation of KLK8 and PKD1 expression in vivo. We found that circSSPO was an oncogenic circRNA that was significantly abundant in ESCC tissues and circSSPO exhibited an oncogenic activity in ESCC by elevating expression of KLK8 and PKD1 through suppressing miR-6820-5p expression.






Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Code Availability
Not applicable.
References
Adamopoulos PG, Tsiakanikas P, Scorilas A. Kallikrein-related peptidases and associated microRNAs as promising prognostic biomarkers in gastrointestinal malignancies. Biol Chem. 2018;399(8):821–36.
Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci. 2020;21(5)
Arnaiz E, Sole C, Manterola L, Iparraguirre L, Otaegui D, Lawrie CH. CircRNAs and cancer: biomarkers and master regulators. Semin Cancer Biol. 2019;58:90–9.
Chen X, Jiang J, Zhao Y, Wang X, Zhang C, Zhuan L, et al. Circular RNA circNTRK2 facilitates the progression of esophageal squamous cell carcinoma through up-regulating NRIP1 expression via miR-140-3p. J Exp Clin Cancer Res. 2020;39(1):133.
Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, et al. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88(3):413–26.
Du WW, Zhang C, Yang W, Yong T, Awan FM, Yang BB. Identifying and characterizing circRNA-protein interaction. Theranostics. 2017;7(17):4183–91.
Fan L, Cao Q, Liu J, Zhang J, Li B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer. 2019;18(1):16.
Feng Q, Zhang H, Yao D, Chen WD, Wang YD. Emerging role of non-coding RNAs in esophageal squamous cell carcinoma. Int J Mol Sci. 2019;21(1)
Grin A, Samaan S, Tripathi M, Rotondo F, Kovacs K, Bassily MN, et al. Evaluation of human tissue kallikrein-related peptidases 6 and 10 expression in early gastroesophageal adenocarcinoma. Hum Pathol. 2015;46(4):541–8.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8.
Hu X, Wu D, He X, Zhao H, He Z, Lin J, et al. circGSK3beta promotes metastasis in esophageal squamous cell carcinoma by augmenting beta-catenin signaling. Mol Cancer. 2019;18(1):160.
Huang E, Fu J, Yu Q, Xie P, Yang Z, Ji H, et al. CircRNA hsa_circ_0004771 promotes esophageal squamous cell cancer progression via miR-339-5p/CDC25A axis. Epigenomics. 2020;12(7):587–603.
Lam AK. Introduction: Esophageal squamous cell carcinoma-current status and future advances. Methods Mol Biol. 2020;2129:1–6.
Li Y, Hua K, Jin J, Fang L. miR-497 inhibits proliferation and invasion in triple-negative breast cancer cells via YAP1. Oncol Lett. 2021;22(2):580.
Liang Y, Mao Q, Wang L, Xia W, Chen B, Wang H, et al. CircIMMP2L promotes esophageal squamous cell carcinoma malignant progression via CtBP1 nuclear retention dependent epigenetic modification. Clin Transl Med. 2021;11(9):e519.
Liou GY, Doppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, et al. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun. 2015;6:6200.
Lopez-Jimenez E, Rojas AM, Andres-Leon E. RNA sequencing and prediction tools for circular RNAs analysis. Adv Exp Med Biol. 2018;1087:17–33.
Lu YF, Yu JR, Yang Z, Zhu GX, Gao P, Wang H, et al. Promoter hypomethylation mediated upregulation of microRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J Exp Clin Cancer Res. 2018;37(1):301.
Martinez-Leon E, Amable G, Jacamo R, Picco ME, Anaya L, Rozengurt E, et al. Protein kinase D1 inhibition interferes with mitosis progression. J Cell Physiol. 2019;234(11):20510–9.
Ng WL, Mohd Mohidin TB, Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol. 2018;15(8):995–1005.
Nohara K, Yamada K, Yamada L, Hagiwara T, Igari T, Yokoi C, et al. Expression of kallikrein-related peptidase 13 is associated with poor prognosis in esophageal squamous cell carcinoma. Gen Thorac Cardiovasc Surg. 2018;66(6):351–7.
Pan Z, Lin J, Wu D, He X, Wang W, Hu X, et al. Hsa_circ_0006948 enhances cancer progression and epithelial-mesenchymal transition through the miR-490-3p/HMGA2 axis in esophageal squamous cell carcinoma. Aging (Albany NY). 2019;11(24):11937–54.
Reichenbach ZW, Murray MG, Saxena R, Farkas D, Karassik EG, Klochkova A, et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res. 2019;144:95–135.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22.
Sang C, Chao C, Wang M, Zhang Y, Luo G, Zhang X. Identification and validation of hub microRNAs dysregulated in esophageal squamous cell carcinoma. Aging (Albany NY). 2020;12(10):9807–24.
Shi Y, Fang N, Li Y, Guo Z, Jiang W, He Y, et al. Circular RNA LPAR3 sponges microRNA-198 to facilitate esophageal cancer migration, invasion, and metastasis. Cancer Sci. 2020;111(8):2824–36.
Sudo K, Kato K, Matsuzaki J, Boku N, Abe S, Saito Y, et al. Development and validation of an esophageal squamous cell carcinoma detection model by large-scale microRNA profiling. JAMA Netw Open. 2019;2(5):e194573.
Sudo N, Ichikawa H, Muneoka Y, Hanyu T, Kano Y, Ishikawa T, et al. Clinical utility of ypTNM stage grouping in the 8th edition of the American Joint Committee on Cancer TNM staging system for esophageal squamous cell carcinoma. Ann Surg Oncol. 2021;28(2):650–60.
Suknovic N, Tomczyk S, Colevret D, Perruchoud C, Galliot B. The ULK1 kinase, a necessary component of the pro-regenerative and anti-aging machinery in Hydra. Mech Ageing Dev. 2021;194:111414.
Yang C, Ding P, Wang Q, Zhang L, Zhang X, Zhao J, et al. Inhibition of complement retards ankylosing spondylitis progression. Sci Rep. 2016;6:34643.
Yoon TW, Fitzpatrick EA, Snyder JD, Lee S, Kim YI, Zacheaus C, et al. Contribution of protein kinase D1 on acute pulmonary inflammation and hypersensitivity pneumonitis induced by Saccharopolyspora rectivirgula. Immunohorizons. 2022;6(3):224–42.
Zheng SR, Huang QD, Zheng ZH, Zhang ZT, Guo GL. circGFRA1 affects the sensitivity of triple-negative breast cancer cells to paclitaxel via the miR-361-5p/TLR4 pathway. J Biochem. 2021;169(5):601–11.
Funding
1. Primary Health Care Foundation of China-Clinical Application Research and Medical Training Fund Project Key Project (No: 2021120002). 2. 2022 Guangzhou Basic Research Plan, basic and applied basic research projects jointly funded by the city and schools (college) (No: 202201020078). 3. Guangdong Science and Technology special fund in 2020 (Shan Fu Ke letter (2020) 53; Project ID: 200113165875501 and 200115105879476). 4. 2022 Guangdong Science and Technology Innovation Strategic Special project (“ Major projects + Task List “) [Shan Fu Ke letter (2022) 124, Project ID: STKJ202209072]. 5. Research-oriented Hospital Program of Guangzhou [RHPG05].
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Qianhua Luo, Junzheng Li, Haixiong Miao, Siman Su, Chengcheng Xu. Performed experimental: Yun Chen, Chengkuan Zhao, Jianxiang Huang. Performed experimental validation: Kai Ling. Analyzed the data and conceived figures and tables: Yun Chen, Chengkuan Zhao, Jianxiang Huang. Wrote the paper: Qianhua Luo, Junzheng Li, Haixiong Miao, Siman Su, Chengcheng Xu. Contributed to the writing of the manuscript: Chaoxian Lin, Hongfei Yan, Shuyao Zhang. All authors revised and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study complied with the ethical guidelines of the Declaration of Helsinki and was approved by the Clinical Ethics Committee of Guangzhou Red Cross Hospital of Jinan University (No. 2021-083-02). All enrolled patients provided written informed consent. All animal experiments were approved by the ethics committee of Guangzhou Red Cross Hospital of Jinan University.
Consent to participate
Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Simple summary: This study was performed to analyze the role of circSSPO in esophageal squamous cell (ESCC).
Supplementary Information
ESM 1
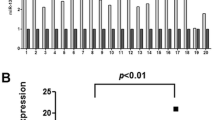
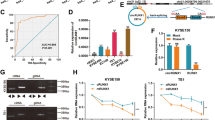
Supplementary Figure 1. Identification of circSSPO. A, The chromosomal positioning and Sanger sequencing results of circSSPO. The black arrow marks the reverse splice point of the circSSPO. B, Agarose gel electrophoresis results of the cDNA and gDNA amplified with divergent and convergent primers in TE-1 and EC9706 cells. C, After the treatment of TE-1 and EC9706 cells with RNase R, levels of linear SSPO mRNA and circSSPO were determined by RT-qPCR. * p < 0.05, compared with the Mock group. D, After the treatment of TE-1 and EC9706 cells with actinomycin D, the abundance of linear SSPO mRNA and circSSPO was measured by RT-qPCR. * p < 0.05, compared with 0 h. Cell experiments were repeated three times independently. (JPG 766 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Q., Li, J., Miao, H. et al. circSSPO boosts growth of esophageal squamous cell carcinoma through upregulation of micrRNA-6820-5p-mediated KLK8 and PKD1 expression. Cell Biol Toxicol 39, 3219–3234 (2023). https://doi.org/10.1007/s10565-023-09828-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-023-09828-3