Abstract
Evodiamine is a major alkaloid component found in the fruit of Evodia rutaecarpa. It shows the anti-proliferative potential against a wide range of cancers by suppressing cell growth, invasion, and metastasis and inducing apoptosis both in vitro and in vivo. Evodiamine shows its anticancer potential by modulating aberrant signaling pathways. Additionally, the review focuses on several therapeutic implications of evodiamine, such as epigenetic modification, cancer stem cells, and epithelial to mesenchymal transition. Moreover, combinatory drug therapeutics along with evodiamine enhances the anticancer efficacy of chemotherapeutic drugs in various cancers by overcoming the chemo resistance and radio resistance shown by cancer cells. It has been widely used in preclinical trials in animal models, exhibiting very negligible side effects against normal cells and effective against cancer cells. The pharmacokinetic and pharmacodynamics-based collaborations of evodiamine are also included. Due to its poor bioavailability, synthetic analogs of evodiamine and its nano capsule have been formulated to enhance its bioavailability and reduce toxicity. In addition, this review summarizes the ongoing research on the mechanisms behind the antitumor potential of evodiamine, which proposes an exciting future for such interests in cancer biology.
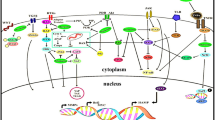
Graphical abstract





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ROS:
-
Reactive oxygen species
- Top I:
-
Topoisomerase I
- CSC:
-
Cancer stem cell
- EMT:
-
Epithelial to mesenchymal transition
- DR:
-
Death receptor
- Akt:
-
Protein kinase B
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B
- SHH:
-
Sonic Hedgehog
- GLI1:
-
GLI family zinc finger 1
- cdk:
-
Cyclin-dependent kinase
- p:
-
Phosphorylated
- chk:
-
Checkpoint kinase
- DNMT:
-
DNA methyl transferase
- Notch3:
-
Neurogenic locus notch homolog protein 3
- mTOR:
-
Mammalian target of rapamycin
- S6K1:
-
Ribosomal protein S6 kinase beta-1
- Mcl-1:
-
Myeloid cell leukemia 1
- PARP :
-
Poly ADP ribose polymerase
- Nbk:
-
Natural born killer
- ER:
-
Estrogen receptor
- MEK:
-
Mitogen-activated protein kinase kinase
- ERK:
-
Extracellular-signal-regulated kinase
- PPAR:
-
Peroxisome proliferator-activated receptor gamma
- STAT3:
-
Signal transducer and activator of transcription 3
- SHP-1:
-
Src homology 2 domain-containing protein tyrosine phosphatase 1
- WWOX:
-
WWdomain-containing oxidoreductase
- VEGF:
-
Vascular endothelial growth factor
- NOD1:
-
Nucleotide-binding oligomerization domain-containing protein 1
- MAPK:
-
Mitogen-activated protein kinase
- YAP:
-
Yes-associated protein
- JNK:
-
C-Jun N-terminal kinase
- JAK2:
-
Janus Kinase 2
- MMP:
-
Matrix metallopeptidase
- Her-2:
-
Human epidermal growth factor receptor 2
- PLK1:
-
Polo-like kinase 1
- Wnt:
-
Wingless-related integration site
- IL:
-
Interleukin
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- NO:
-
Nitric oxide
- Mcl-1:
-
Myeloid cell leukemia 1
- SIRT1:
-
Sirtuin
- PKB:
-
Protein kinase B
- TGFβ:
-
Transforming growth factor beta
- Smad2:
-
Mothers against decapentaplegic homolog 2
- GADD45:
-
Growth arrest and DNA damage-inducible 45
- Mcm:
-
Minichromosome maintenance complex component
- TRADD:
-
Tumor necrosis factor receptor type 1-associated DEATH domain
- IRAK:
-
Interleukin-1 receptor-associated kinase
- cyt-c:
-
Cytochrome-c
- AIF:
-
Apoptosis-inducing factor
- ENDOG:
-
Endonuclease G
- c-Met:
-
Tyrosine-protein kinase Met
- Src:
-
Sarcoma
- Myt-1:
-
Myelin transcription factor 1
- miR:
-
MicroRNA
- NSCLC:
-
Non-small cell lung cancer
- PRAME:
-
Preferentially expressed antigen of melanoma
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- IGF-1:
-
Insulin-like growth factor
- PTEN:
-
Phosphatase and tensin homolog
- cAMP:
-
Cyclic adenosine monophosphate
- TRAIL:
-
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand
- uPA:
-
Urokinase-type plasminogen activator
- COX-2:
-
Cyclooxygenase-2
- ICAM-1:
-
Intercellular cell adhesion molecule-1
- MDR:
-
Multidrug resistance
- XIAP:
-
X-linked inhibitor of apoptosis protein
- IAP:
-
Inhibitor of apoptosis protein
- FLIP:
-
Fas‐associated death domain (FADD)‐like IL‐1β‐converting enzyme‐inhibitory protein
- IkBα:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- TNF:
-
Tumor necrosis factor
- IKK:
-
IκB kinase
- ABCG2:
-
ATP-binding cassette super-family G member 2
- LPS:
-
Lipopolysaccharide
- iNOS:
-
Inducible NOS
- CCA:
-
Cholangiocarcinoma
- PGI:
-
Phosphoglucose isomerase
- AMF:
-
Autocrine motility factor
- SHP:
-
Phosphatase shatterproof 1 N-terminal
- NBD:
-
Nucleotide binding domain
- HSP70:
-
Heat shock protein
- Sox2:
-
Sex determining region Y)-box 2
- KLF4:
-
Kruppel-like factor 4
- Oct4:
-
Octamer-binding transcription factor 4
- LRP5:
-
Low-density lipoprotein receptor-related protein 5
- SCD1:
-
Stearoyl-CoA desaturase
- HER:
-
Human epidermal growth factor receptor
- hes-5:
-
Hes family BHLH transcription factor 5
- Mst1/2:
-
Mammalian sterile 20-like kinase (MST) 1/2
- MET:
-
Mesenchymal to epithelial transition
- HGF:
-
Human growth factor
- TGF-β:
-
Transforming growth factor beta
- 5-aza:
-
5-Azacytidine
- TSA:
-
Trichostatin A
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- L-OHP:
-
Oxaliplatin
- HCT-116/L-OHP:
-
L-OHP resistant HCT-116
- CCRF-CEM/C1:
-
Camptothecin-resistant leukemic lymphoblast cells
- A2780R2000 :
-
Camptothecin-resistant A2780
- A2780/PTXR :
-
Paclitaxel resistant A2780
- ZJW:
-
Zuo-Jin-Wan
- 5-FU:
-
5-Fluorouracil
- Pt:
-
Platinum
- ERCC1:
-
Excision repair cross-complementing 1
- TS:
-
Thymidylate synthase
- HDAC:
-
Histone deacetylase
- H2AX:
-
H2A histone family member X
- DOX:
-
Doxorubicin
- LD50:
-
Lethal dose, 50%
- IC50 :
-
Inhibitory concentration, 50%
- T/NT ratio:
-
Tumor:nontumor ratio
- SUV:
-
Standardized uptake value
- AFP:
-
α-Fetoprotein
- CRC:
-
Colorectal cancer
- TSGF:
-
Tumor specific growth factor
- NEEPN:
-
Evodiamine-phospholipid nano complex
- MSNs:
-
Mesoporous silica nanoparticles
References
Bak E, et al. Inhibitory effect of evodiamine alone and in combination with rosiglitazone on in vitro adipocyte differentiation and in vivo obesity related to diabetes. Int J Obes. 2010;34(2):250–60.
Barrero MJ, Boué S, Belmonte JCI. Epigenetic mechanisms that regulate cell identity. Cell Stem Cell. 2010;7(5):565–70.
Bhatia K, Das A. Combinatorial drug therapy in cancer-new insights. Life Sciences. 2020;118134.
Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233.
Burotto M, et al. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120(22):3446–56.
Cai Q, et al. Toxicity of Evodiae fructus on rat liver mitochondria: the role of oxidative stress and mitochondrial permeability transition. Molecules. 2014;19(12):21168–82.
Chan AL-F, et al. Evodiamine stabilizes topoisomerase I-DNA cleavable complex to inhibit topoisomerase I activity. Molecules. 2009;14(4):1342–52.
Chandarlapaty S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2012;2(4):311–9.
Chang W-L, et al. Tubulin-binding agents down-regulate matrix metalloproteinase-2 and-9 in human hormone-refractory prostate cancer cells–a critical role of cdk1 in mitotic entry. Biochem Pharmacol. 2015;94(1):12–21.
Chen MC, et al. Anti-proliferative effects of evodiamine on human thyroid cancer cell line ARO. J Cell Biochem. 2010;110(6):1495–503.
Chen T-C, et al. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine. 2016;23(1):68–78.
Cheng ZM, et al. Effects of evodiamine on invasion and polo-like kinase 1 expression of human gastric cancer cell [J]. J Jiangsu Univ (Medicine Edition) 2011;21:69–72.
Chien C-C, et al. Activation of JNK contributes to evodiamine-induced apoptosis and G 2/M arrest in human colorectal carcinoma cells: a structure-activity study of evodiamine. PLoS One. 2014;9(6):e99729.
Chou S-T, et al. Exploration of anti-cancer effects and mechanisms of Zuo-Jin-Wan and its alkaloid components in vitro and in orthotopic HepG2 xenograft immunocompetent mice. BMC Complement Altern Med. 2017;17(1):121.
Cohen SM, et al. Hepatotoxicity associated with the use of White Flood, a nutritional supplement. Pract Gastroenterol. 2012;36(10):45–7.
Dong G, et al. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J Med Chem. 2010;53(21):7521–31.
Dong G, et al. New tricks for an old natural product: discovery of highly potent evodiamine derivatives as novel antitumor agents by systemic structure–activity relationship analysis and biological evaluations. 2012;55(17):7593–613.
Du J, et al. Evodiamine induces apoptosis and inhibits metastasis in MDA-MB-231 human breast cancer cells in vitro and in vivo. Oncol Rep. 2013;30(2):685–94.
Du J, et al. Berberine and evodiamine act synergistically against human breast cancer MCF-7 cells by inducing cell cycle arrest and apoptosis. Anticancer Res. 2017;37(11):6141–51.
Duchartre Y, Kim Y-M, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–9.
Ebi H. EGFR-Mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition. Ann Oncol. 2012;23:xi41.
Fang C, et al. Evodiamine induces G2/M arrest and apoptosis via mitochondrial and endoplasmic reticulum pathways in H446 and H1688 human small-cell lung cancer cells. PLoS One. 2014;9(12):e115204.
Fang Q, et al. Evodiamine selectively inhibits multiple myeloma cell growth by triggering activation of intrinsic apoptosis pathway. Onco Targets Ther. 2019;12:11383.
Fendler A, et al. Inhibiting WNT and NOTCH in renal cancer stem cells and the implications for human patients. Nat Commun. 2020;11(1):1–16.
García-Gómez R, Bustelo XR, Crespo P. Protein–protein interactions: emerging oncotargets in the RAS-ERK pathway. Trends Cancer. 2018;4(9):616–33.
Gu Y, Mohammad IS, Liu Z. Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol Lett. 2020;19(4):2585–94.
Guan X, et al. Combined effects of berberine and evodiamine on colorectal cancer cells and cardiomyocytes in vitro. Eur J Pharmacol 2020;173031.
Guo X-X, et al. Evodiamine induces apoptosis in SMMC-7721 and HepG2 cells by suppressing NOD1 signal pathway. Int J Mol Sci. 2018;19(11):3419.
Guo Q, et al. Evodiamine inactivates NF-κB and potentiates the antitumor effects of gemcitabine on tongue cancer both in vitro and in vivo. Onco Targets Ther. 2019;12:257.
Guo YJ, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007.
Gupta SC, et al. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta (BBA)-Gene Regul Mech. 2010;1799(10–12):775–87.
Han S, et al. Evodiamine selectively targets cancer stem-like cells through the p53–p21-Rb pathway. Biochem Biophys Res Commun. 2016;469(4):1153–8.
He C, Wan H. Drug metabolism and metabolite safety assessment in drug discovery and development. Expert Opin Drug Metab Toxicol. 2018;14(10):1071–85.
Heo SK, et al. Evodiamine and rutaecarpine inhibit migration by LIGHT via suppression of NADPH oxidase activation. J Cell Biochem. 2009;107(1):123–33.
Hong Z, et al. Effects of evodiamine on PI3K/Akt and MAPK/ERK signaling pathways in pancreatic cancer cells. Int J Oncol. 2020;56(3):783–93.
Hu J, et al. Improved absorption and in vivo kinetic characteristics of nanoemulsions containing evodiamine–phospholipid nanocomplex. Int J Nanomed. 2014;9:4411.
Hu C, et al. Evodiamine sensitizes BGC-823 gastric cancer cells to radiotherapy in vitro and in vivo. Mol Med Rep. 2016;14(1):413–9.
Hu C-Y, et al. Evodiamine exerts an anti-hepatocellular carcinoma activity through a WWOX-dependent pathway. Molecules. 2017;22(7):1175.
Hu X, et al. Antiproliferative effects of alkaloid evodiamine and its derivatives. Int J Mol Sci. 2018;19(11):3403.
Huang Y-C, Guh J-H, Teng C-M. Induction of mitotic arrest and apoptosis by evodiamine in human leukemic T-lymphocytes. Life Sci. 2004;75(1):35–49.
Huang J, et al. Antiproliferation effect of evodiamine in human colon cancer cells is associated with IGF-1/HIF-1α downregulation. Oncol Rep. 2015;34(6):3203–11.
Huang C, et al. Effect of evodiamine and berberine on the interaction between DNMTs and target microRNAs during malignant transformation of the colon by TGF-β1. Oncol Rep. 2017;37(3):1637–45.
Hung P-H, et al. Inhibitory effect of evodiamine on aldosterone release by Zona glomerulosa cells in male rats. Chin J Physiol. 2001;44(2):53–8.
Hwang ST, et al. Evodiamine mitigates cellular growth and promotes apoptosis by targeting the c-met pathway in prostate cancer cells. Molecules. 2020;25(6):1320.
Hyun SY, et al. Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics. 2021;11(6):2932.
Jia J, et al. Inhibition of human liver cancer cell growth by evodiamine involves apoptosis and deactivation of PI3K/AKT pathway. Appl Biol Chem. 2020;63(1):1–8.
Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14(5):1852–9.
Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial–mesenchymal transition. Cells. 2020;9(1):217.
Jin J, et al. Identification of genetic mutations in cancer: challenge and opportunity in the new era of targeted therapy. Front Oncol. 2019;9:263.
Junlin L, et al. The effects of evodiamine on autophagy in human colon adenocarcinoma lovo cells. Chin J Gen Surg. 2011;26(1):41–4.
Kabacaoglu D, et al. NF-κB/Rel transcription factors in pancreatic cancer: focusing on RelA, c-Rel, and RelB. Cancers. 2019;11(7):937.
Kan SF, et al. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int J Cancer. 2004;110(5):641–51.
Kan SF, et al. Anti-proliferative effects of evodiamine on human prostate cancer cell lines DU145 and PC3. J Cell Biochem. 2007;101(1):44–56.
Khan M, et al. Evodiamine sensitizes U87 glioblastoma cells to TRAIL via the death receptor pathway. Mol Med Rep. 2015;11(1):257–62.
Kharkar PS. Cancer stem cell (CSC) inhibitors: a review of recent patents (2012–2015). Expert Opin Ther Pat. 2017;27(7):753–61.
Kim H-J, et al. Effect of herbal Ephedra sinica and Evodia rutaecarpa on body composition and resting metabolic rate: a randomized, double-blind clinical trial in Korean premenopausal women. J Acupunct Meridian Stud. 2008;1(2):128–38.
Kim SH, et al. Evodiamine suppresses survival, proliferation, migration and epithelial–mesenchymal transition of thyroid carcinoma cells. Anticancer Res. 2018;38(11):6339–52.
Kim H, et al. Evodiamine eliminates colon cancer stem cells via suppressing Notch and Wnt signaling. Molecules. 2019a;24(24):4520.
Kim SH, et al. Evodiamine in combination with histone deacetylase inhibitors has synergistic cytotoxicity in thyroid carcinoma cells. Endocrine. 2019b;65(1):110–20.
Komatsu K-I, Wakame K, Kano Y. Pharmacological properties of galenical preparation. XVI. Pharmacokinetics of evodiamine and the metabolite in rats. Biol Pharm Bull. 1993;16(9):935–8.
Lee T-J, et al. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol Cancer Ther. 2006;5(9):2398–407.
Lee Y-C, et al. Targeting of topoisomerase I for prognoses and therapeutics of camptothecin-resistant ovarian cancer. PLoS One. 2015;10(7):e0132579.
Li L, et al. Microbial metabolism of evodiamine by Penicillium janthinellum and its application for metabolite identification in rat urine. Enzyme Microb Technol. 2006;39(4):561–7. https://doi.org/10.1016/j.enzmictec.2005.10.029.
Li HL, et al. MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo. Mol Med Rep. 2016a;14(2):1430–8.
Li Y-L, et al. Evodiamine induces apoptosis and enhances apoptotic effects of erlotinib in wild-type EGFR NSCLC cells via S6K1-mediated Mcl-1 inhibition. Med Oncol. 2016b;33(2):16.
Li Y-L, et al. Evodiamine induces apoptosis and promotes hepatocellular carcinoma cell death induced by vorinostat via downregulating HIF-1α under hypoxia. Biochem Biophys Res Commun. 2018;498(3):481–6.
Li C, et al. Development of EGFR-targeted evodiamine nanoparticles for the treatment of colorectal cancer. Biomater Sci. 2019;7(9):3627–39.
Li FS, et al. BMP9 mediates the anticancer activity of evodiamine through HIF-1α/p53 in human colon cancer cells. Oncol Rep. 2020;43(2):415–26.
Liao C-H, et al. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis. 2005;26(5):968–75.
Liao J-F, et al. Anti-inflammatory and anti-infectious effects of Evodia rutaecarpa (Wuzhuyu) and its major bioactive components. Chin Med. 2011;6(1):6.
Lijuan W, et al. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase B signaling pathways. J Tradit Chin Med. 2016;36(3):353–9.
Lin C, et al. Simultaneous determination of evodiamine and rutecarpine in rabbit plasma by LC-ESI-MS and its application to pharmacokinetics. Die Pharmazie-an Int J Pharm Sci. 2011;66(12):920–3.
Lin L, et al. Effect of evodiamine on the proliferation and apoptosis of A549 human lung cancer cells. Mol Med Rep. 2016;14(3):2832–8.
Lin H, et al. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int J Pharm 2020;119278.
Liu A-J, et al. Evodiamine, a plant alkaloid, induces calcium/JNK-mediated autophagy and calcium/mitochondria-mediated apoptosis in human glioblastoma cells. Chem Biol Interact. 2013;205(1):20–8.
Liu H, et al. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Onco Targets Ther. 2016;9:4121.
Luo C, et al. Research progress on evodiamine, a bioactive alkaloid of Evodiae fructus: focus on its anti-cancer activity and bioavailability. Exp Ther Med. 2021;22(5):1–11.
Meng Z-J, et al. Evodiamine inhibits the proliferation of human osteosarcoma cells by blocking PI3K/Akt signaling. Oncol Rep. 2015;34(3):1388–96.
Mohan V, Agarwal R, Singh RP. A novel alkaloid, evodiamine causes nuclear localization of cytochrome-c and induces apoptosis independent of p53 in human lung cancer cells. Biochem Biophys Res Commun. 2016;477(4):1065–71.
Mondal S, et al. Natural products: promising resources for cancer drug discovery. Anti-Cancer Agents Med Chem (Formerly Curr Med Chem-Anti-Cancer Agents). 2012;12(1):49–75.
Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16(8):797–803.
Morrison R, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011;2011
Myung JK, et al. Bioavailability enhancing activities of natural compounds from medicinal plants. J Med Plants Res. 2009;3(13):1204–11.
Noorolyai S, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–8.
Ogasawara M, Suzuki H. Inhibition by evodiamine of hepatocyte growth factor-induced invasion and migration of tumor cells. Biol Pharm Bull. 2004;27(4):578–82.
Ogasawara M, Matsubara T, Suzuki H. Inhibitory effects of evodiamine on in vitro invasion and experimental lung metastasis of murine colon cancer cells. Biol Pharm Bull. 2001;24(8):917–20.
O’Shea JJ, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28.
Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 2018;47(19):6645–53.
Pan X, et al. Evodiamine, a dual catalytic inhibitor of type I and II topoisomerases, exhibits enhanced inhibition against camptothecin resistant cells. Phytomedicine. 2012;19(7):618–24.
Panda M, Biswal BK. Cell signaling and cancer: a mechanistic insight into drug resistance. Mol Biol Rep. 2019;46(5):5645–59.
Panda M, Biswal BK. Evodiamine inhibits stemness and metastasis by altering the SOX9–β‐catenin axis in non‐small‐cell lung cancer. J Cell Biochem. 2022.
Panda M, Tripathi SK, Biswal BK. Plumbagin promotes mitochondrial mediated apoptosis in gefitinib sensitive and resistant A549 lung cancer cell line through enhancing reactive oxygen species generation. Mol Biol Rep. 2020;47:4155–68.
Panda M, Tripathi SK, Biswal BK. SOX9: an emerging driving factor from cancer progression to drug resistance. Biochim Biophys Acta (BBA)-Rev Cancer. 2021;188517.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–26.
Patel M, et al. NF-κB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56.
Peng X, et al. Evodiamine inhibits the migration and invasion of nasopharyngeal carcinoma cells in vitro via repressing MMP-2 expression. Cancer Chemother Pharmacol. 2015;76(6):1173–84.
Qi W, Zhou Y, Fan Y. Effects of evodiamine on invasion and proliferation in human gastric cancer cell. Shandong Med J. 2010;34:016.
Qiu C, et al. A promising antitumor activity of evodiamine incorporated in hydroxypropyl-β-cyclodextrin: pro-apoptotic activity in human hepatoma HepG2 cells. Chem Cent J. 2016;10(1):1–11.
Ramli S, et al. Long noncoding RNA UCA1 in gastrointestinal cancers: molecular regulatory roles and patterns, mechanisms, and interactions. J Oncol. 2021;2021.
Sachita K, et al. In vitro assessment of the anticancer potential of evodiamine in human oral cancer cell lines. Phytother Res. 2015;29(8):1145–51.
Schnekenburger M, Dicato M, Diederich MF. Anticancer potential of naturally occurring immunoepigenetic modulators: a promising avenue? Cancer. 2019;125(10):1612–28.
Seshacharyulu P, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):15–31.
Shakil MS, et al. In vivo toxicity studies of chitosan-coated cobalt ferrite nanocomplex for its application as MRI contrast dye. 2020;3(11):7952-7964.
Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36.
Shen H, et al. Evodiamine inhibits proliferation and induces apoptosis in gastric cancer cells. Oncol Lett. 2015;10(1):367–71.
Sheng C, Miao Z, Zhang WJSINPC. Topoisomerase I inhibitors derived from natural products: structure–activity relationships and antitumor potency. Stud Nat Prod Chem. 2016;47:1–28.
Shi H-L, et al. Berberine counteracts enhanced IL-8 expression of AGS cells induced by evodiamine. Life Sci. 2013;93(22):830–9.
Shi L, et al. Evodiamine exerts anti-tumor effects against hepatocellular carcinoma through inhibiting β-catenin-mediated angiogenesis. Tumor Biology. 2016;37(9):12791–803.
Shi C-S, et al. Evodiamine induces cell growth arrest, apoptosis and suppresses tumorigenesis in human urothelial cell carcinoma cells. Anticancer Res. 2017a;37(3):1149–59.
Shi PX, et al. Evodiamine suppresses proliferation of colon cancer HCT-116 cells in mice. Basic Clin Med. 2017b;37(10):1373–7.
Shyr M-H, et al. Determination and pharmacokinetics of evodiamine in the plasma and feces of conscious rats. Anal Chim Acta. 2006;558(1–2):16–21.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51.
Siveen KS, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta (BBA)-Rev Cancer. 2014;1845(2):136–54.
Su T, et al. Evodiamine, a novel NOTCH3 methylation stimulator, significantly suppresses lung carcinogenesis in vitro and in vivo. Front Pharmacol. 2018;9:434.
Sui H, et al. Evodiamine suppresses ABCG2 mediated drug resistance by inhibiting p50/p65 NF-κB pathway in colorectal cancer. J Cell Biochem. 2016;117(6):1471–81.
Sun HZ, et al. Investigation of the in vitro metabolism of evodiamine: characterization of metabolites and involved cytochrome p450 isoforms. Phytother Res. 2013;27(5):705–12. https://doi.org/10.1002/ptr.4766.
Sun C, et al. Evodiamine inhibits the proliferation of leukemia cell line K562 by regulating peroxisome proliferators-activated receptor gamma (PPAR γ) pathway. J Recept Signal Transduction. 2016;36(4):422–8.
Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-κB activation by inhibiting IκBα kinase activation, thereby suppressing NF-κB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280(17):17203–12.
Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141(2):140–9.
Takebe N, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12(8):445.
Tan Q, et al. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS PharmSciTech. 2012;13(2):534–47.
Tan Q, Zhang J. Evodiamine and its role in chronic diseases. Drug Discov Mother Nat 2016;315–328.
Tanabe S, et al. Interplay of EMT and CSC in cancer and the potential therapeutic strategies. Front Pharmacol. 2020;11:904.
Teschke R. Traditional Chinese Medicine induced liver injury. J Clin Transl Hepatol. 2014;2(2):80.
Thu KL, et al. Genetic disruption of KEAP1/CUL3 E3 ubiquitin ligase complex components is a key mechanism of NF-kappaB pathway activation in lung cancer. J Thorac Oncol. 2011;6(9):1521–9.
Tripathi SK, Biswal BK. Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol Res. 2020;156:104772.
Tripathi SK, Panda M, Biswal BK. Emerging role of plumbagin: cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem Toxicol. 2019;125:566–82.
Tripathi SK, et al. Recent updates on the resistance mechanisms to epidermal growth factor receptor tyrosine kinase inhibitors and resistance reversion strategies in lung cancer. Med Res Rev. 2020;40(6):2132–76.
Tu Y-J, et al. Evodiamine activates autophagy as a cytoprotective response in murine Lewis lung carcinoma cells. Oncol Rep. 2013;29(2):481–90.
Tune BXJ, et al. Matrix metalloproteinases in chemoresistance: regulatory roles, molecular interactions, and potential inhibitors. J Oncol. 2022;2022.
Wang C, et al. Effect of protein kinase C on human melanoma A375–S2 cell death induced by evodiamine. Yao Xue Xue Bao= Acta Pharmaceutica Sinica. 2005;40(11):1033–6.
Wang C, et al. Roles of SIRT1 and phosphoinositide 3-OH kinase/protein kinase C pathways in evodiamine-induced human melanoma A375–S2 cell death. J Pharmacol Sci. 2005a;97(4):494–500.
Wang C, et al. Evodiamine induced human melanoma A375–S2 cell death partially through interleukin 1 mediated pathway. Biol Pharm Bull. 2005b;28(6):984–9.
Wang X-N, et al. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine. 2008;15(12):1062–8.
Wang C, Li S, Wang M-W. Evodiamine-induced human melanoma A375–S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-κB signaling pathways and augmented by ubiquitin–proteasome inhibition. Toxicol Vitro. 2010;24(3):898–904.
Wang S, Chen M, Wang Y. Tumor suppressive activity of evodiamine in breast cancer cells via inhibition of Ras/MEK/ERK pathway and activation of PPARγ. Planta Medica. 2012;78(05):P_79.
Wang K-L, et al. Anti-proliferative effects of evodiamine on human breast cancer cells. PLoS One. 2013;8(6):e67297.
Wang S, et al. Evodiamine synergizes with doxorubicin in the treatment of chemoresistant human breast cancer without inhibiting P-glycoprotein. PLoS One. 2014;9(5):e97512.
Wang P, et al. Overcome cancer cell drug resistance using natural products. Evid-Based Complement Alternat Med 2015;2015.
Wang R, et al. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7(5):5754.
Wang R, et al. Evodiamine activates cellular apoptosis through suppressing PI3K/AKT and activating MAPK in glioma. Onco Targets Ther. 2018a;11:1183.
Wang CY, et al. Simultaneous determination of evodiamine and its four metabolites in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Biomed Chromatogr 2018b;32(7):e4219.ARTN e4219. https://doi.org/10.1002/bmc.4219.
Wang D, et al. Evodiamine exerts anticancer effects via induction of apoptosis and autophagy and suppresses the migration and invasion of human colon cancer cells. J Bu On:Off J Balkan Union Oncol. 2019;24(5):1824–9.
Wei W-T, et al. Enhanced antitumor efficacy of gemcitabine by evodiamine on pancreatic cancer via regulating PI3K/Akt pathway. Int J Biol Sci. 2012;8(1):1.
Wen B, et al. Metabolic activation of the indoloquinazoline alkaloids evodiamine and rutaecarpine by human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2014;42(6):1044–54.
Wen Z, et al. Evodiamine, a novel inhibitor of the Wnt pathway, inhibits the self-renewal of gastric cancer stem cells. Int J Mol Med. 2015;36(6):1657–63.
Wijdeven RH, et al. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist Updates. 2016;28:65–81.
Wu FP, et al. Profiling and identification of the metabolites of evodiamine in rats using ultra-performance liquid chromatography with linear ion trap orbitrap mass spectrometer. Trop J Pharm Res. 2016a;15(3):623–9. https://doi.org/10.4314/tjpr.v15i3.26.
Wu W-S, et al. Protein kinase RNA-like endoplasmic reticulum kinase-mediated Bcl-2 protein phosphorylation contributes to evodiamine-induced apoptosis of human renal cell carcinoma cells. PLoS One. 2016b;11(8):e0160484.
Wu W-S, et al. Evodiamine prevents glioma growth, induces glioblastoma cell apoptosis and cell cycle arrest through JNK activation. Am J Chin Med. 2017;45(04):879–99.
Xia Y-Y, et al. Simultaneous determination of evodiamine and evodine in Beagle dog plasma using liquid chromatography tandem mass spectrometry. J Asian Nat Prod Res. 2013;15(3):235–43.
Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2(9):823–30.
Xu H, et al. Preparation of evodiamine solid dispersions and its pharmacokinetics. Indian J Pharm Sci. 2011;73(3):276.
Xu S, et al. Pharmacokinetic comparisons of rutaecarpine and evodiamine after oral administration of Wu-Chu-Yu extracts with different purities to rats. J Ethnopharmacol. 2012;139(2):395–400.
Yan S, et al. Difference and alteration in pharmacokinetic and metabolic characteristics of low-solubility natural medicines. Drug Metab Rev. 2018;50(2):140–60.
Yang X-W, et al. Studies on the alkaloid constituents of Evodia rutaecarpa (Juss) Benth var. bodinaieri (Dode) Huang and their acute toxicity in mice. J Asian Nat Prod Res. 2006;8(8):697–703.
Yang J, et al. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radical Res. 2008a;42(5):492–504.
Yang J, et al. Nitric oxide activated by p38 and NF-κ B facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375–S2 cells. Free Radical Res. 2008b;42(1):1–11.
Yang ZG, Chen AQ, Liu B. Antiproliferation and apoptosis induced by evodiamine in human colorectal carcinoma cells (COLO-205). Chem Biodivers. 2009;6(6):924–33.
Yang J, et al. Evodiamine inhibits STAT3 signaling by inducing phosphatase shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett. 2013;328(2):243–51.
Yang K, et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. 2016a;96(2):116-136
Yang K, et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. Lab Invest. 2016b;96(2):116–36.
Yang F, et al. Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017a;485(1):54–61.
Yang W, et al. Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules. 2017b;22(6):943.
Yang X, et al. Head and neck cancers promote an inflammatory transcriptome through coactivation of classic and alternative NF-κB pathways. Cancer Immunol Res. 2019;7(11):1760–74.
Yang S, et al. Evodiamine exerts anticancer effects against 143B and MG63 cells through the Wnt/β-catenin signaling pathway. Cancer Manag Res. 2020a;12:2875.
Yang X, et al. Evodiamine suppresses Notch3 signaling in lung tumorigenesis via direct binding to γ-secretases. Phytomedicine. 2020b;68:153176.
Yu H, et al. Pharmacological actions of multi-target-directed evodiamine. Molecules. 2013;18(2):1826–43.
Yuan X, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369(1):20–7.
Yuan R, et al. Natural products to prevent drug resistance in cancer chemotherapy: a review. Ann N Y Acad Sci. 2017a;1401(1):19–27.
Yuan X-L, et al. Cytological assessments and transcriptome profiling demonstrate that evodiamine inhibits growth and induces apoptosis in a renal carcinoma cell line. Sci Rep. 2017b;7(1):1–10.
Yuan Y, et al. Evodiamine inhibits apoptosis of human osteosarcoma MG-63 cells by blocking wnt/β-catenin signaling. J Int Oncol. 2017c;44(2):86.
Yue G, et al. Synergistic anticancer effects of polyphyllin I and evodiamine on freshly-removed human gastric tumors. PLoS One. 2013;8(6):e65164.
Zhang Y, et al. Intracellular regulation of evodiamine-induced A375–S2 cell death. Biol Pharm Bull. 2003;26(11):1543–7.
Zhang Y, et al. Evodiamine induces tumor cell death through different pathways: apoptosis and necrosis. Acta Pharmacol Sin. 2004;25(1):83.
Zhang C, et al. Evodiamine induces caspase-dependent apoptosis and S phase arrest in human colon lovo cells. Anticancer Drugs. 2010;21(8):766–76.
Zhang T, et al. Evodiamine induces apoptosis and enhances TRAIL-induced apoptosis in human bladder cancer cells through mTOR/S6K1-mediated downregulation of Mcl-1. Int J Mol Sci. 2014;15(2):3154–71.
Zhang J, Tian X-J, Xing J. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J Clin Med. 2016a;5(4):41.
Zhang X, et al. A preliminary study of pharmacokinetics of evodiamine hydroxypropyl-β-cyclodextrin inclusion complex. J South Med Univ. 2016b;36(4):548–51.
Zhang YT, et al. Effect of evodiamine on CYP enzymes in rats by a cocktail method. Pharmacology. 2016c;97(5–6):218–23. https://doi.org/10.1159/000443178.
Zhang Y, Zhang Q, Chen H. BCL9 promotes epithelial mesenchymal transition and invasion in cisplatin resistant NSCLC cells via β-catenin pathway. Life Sci. 2018a;208:284–94.
Zhang Z, et al. Characterization of the in vitro metabolic profile of evodiamine in human liver microsomes and hepatocytes by UHPLC-Q exactive mass spectrometer. Front Pharmacol. 2018b;9:130. https://doi.org/10.3389/fphar.2018.00130.
Zhao L-C, et al. Evodiamine induces apoptosis and inhibits migration of HCT-116 human colorectal cancer cells. Int J Mol Sci. 2015;16(11):27411–21.
Zhao L, et al. Modulatory effect of evodiamine on JAK2/STAT3 signal pathway in HCT-116 cells. Chin Pharm Bull 2015;(10):1394–1397, 1398.
Zhao S, et al. Evodiamine inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells via the Hippo-yes-associated protein signaling pathway. Life Sci. 2020;251:117424.
Zheng Y, et al. Evodiamine induces apoptosis in pancreatic carcinoma PANC-1 cells via NF-κB inhibition. ||| Bangladesh J Pharmacol. 2013;8(1):8–14.
Zhong Z-F, et al. Anti-proliferative activity and cell cycle arrest induced by evodiamine on paclitaxel-sensitive and-resistant human ovarian cancer cells. Sci Rep. 2015;5:16415.
Zhou Y, Hu J. Evodiamine induces apoptosis, G2/M cell cycle arrest, and inhibition of cell migration and invasion in human osteosarcoma cells via Raf/MEK/ERK signalling pathway. Med Sci Monit: Int Med J Exp Clin Res. 2018;24:5874.
Zhou P, et al. Evodiamine inhibits migration and invasion by Sirt1-mediated post-translational modulations in colorectal cancer. Anticancer Drugs. 2019;30(6):611.
Zhu B, et al. Induction of phosphatase shatterproof 2 by evodiamine suppresses the proliferation and invasion of human cholangiocarcinoma. Int J Biochem Cell Biol. 2019a;108:98–110.
Zhu H, et al. Growth inhibitor of human hepatic carcinoma HepG2 cells by evodiamine is associated with downregulation of PRAME. Naunyn Schmiedebergs Arch Pharmacol. 2019b;392(12):1551–60.
Zou Y, et al. Apoptosis of human non-small-cell lung cancer A549 cells triggered by evodiamine through MTDH-dependent signaling pathway. Tumor Biology. 2015;36(7):5187–93.
Zou L, et al. Preparation, characterization, and anticancer efficacy of evodiamine-loaded PLGA nanoparticles. Drug Delivery. 2016;23(3):898–906.
Acknowledgements
The authors acknowledge the National Institute of Technology Rourkela, Odisha, India, for providing laboratory and other facilities to carry out this work.
Funding
Department of Science and Technology, Science and Engineering Research Board (DST, SERB), New Delhi, India (Grant Number: ECR/2016/000792) and Ministry of human resource and development (MHRD), New Delhi, and Department of Science and Technology, Odisha, India (Grant No-1201).
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was prepared by MP. The bioavailability and pharmacokinetics section and Fig. 1 of this manuscript were written and drawn by SKT, and GZ edited the manuscript. BKB guided and edited the manuscript. Lastly, all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors agreed to publish this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panda, M., Tripathi, S.K., Zengin, G. et al. Evodiamine as an anticancer agent: a comprehensive review on its therapeutic application, pharmacokinetic, toxicity, and metabolism in various cancers. Cell Biol Toxicol 39, 1–31 (2023). https://doi.org/10.1007/s10565-022-09772-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-022-09772-8